- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
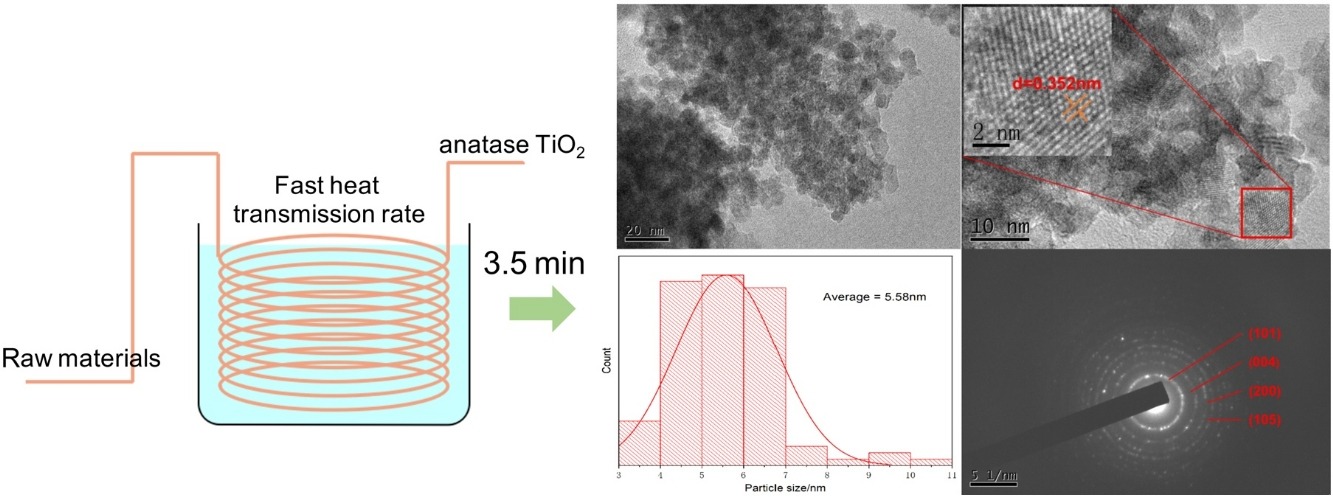
• Fast and continuous synthesis of anatase TiO2 nanoparticles in 3.5 min.
• Simply using a homemade microfluidic system consisted of stainless steel tube.
• Utilizing cheap and environment-friendly inorganic raw materials.
• Particle sizes as small as about 5 nm and specific surface area up to 330 m2/g.
• High photocatalytic performance with the maximum rate 2.1 times of commercial P5.
Previously we had developed a microfluidic system that can be easily fabricated by bending a stainless-steel tube into large circular loops. In this study, a fast and continuous preparation method for superfine TiO2 nanoparticles (TiO2-NPs) was developed for the aforementioned microfluidic system. The proposed method can yield anatase TiO2 in 3.5 min, in contrast to the traditional hydrothermal reaction method, which requires hours or even days. Different reaction conditions, such as reaction temperature (120–200 °C), urea concentration (20–100 g/L), and tube length (5–20 m) were investigated. X-ray diffraction and Brunauer–Emmett–Teller analysis indicate that the as-prepared TiO2-NPs have crystalline sizes of 4.1–5.8 nm and specific surface areas of 250.7–330.7 m2/g. Transmission electron microscopy images show that these TiO2-NPs have an even diameter of approximately 5 nm. Moreover, because of their small crystalline sizes and large specific surface areas, most of these as-prepared TiO2-NPs exhibit considerably better absorption and photocatalytic performance with methylene blue than commercial P5 TiO2 does.