- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
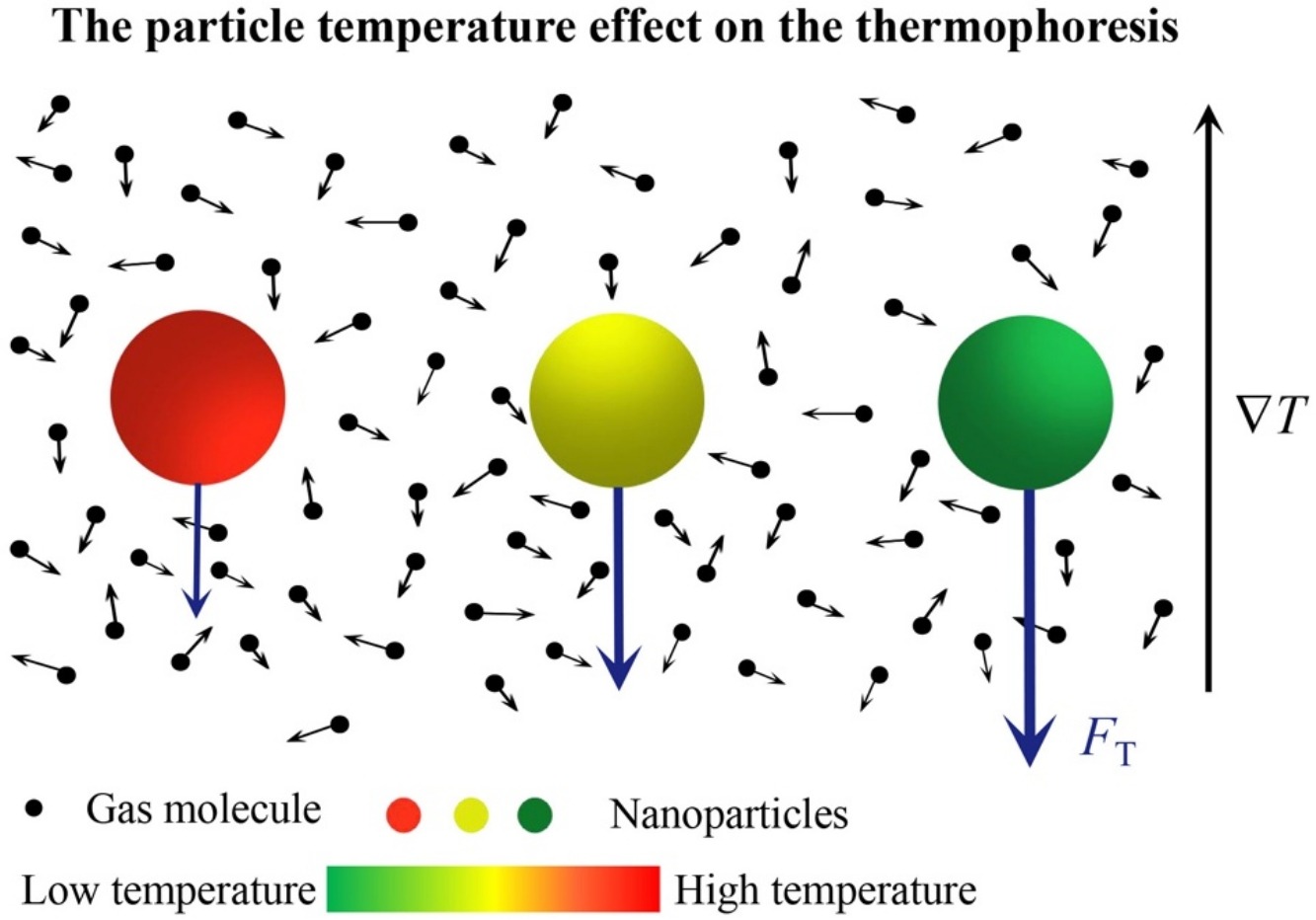
• Particle temperature effect on thermophoresis of nanoparticles is studied.
• Theoretical formulas for the thermophoresis on nanoparticles are obtained.
• Error due to equal gas–particle temperature assumption can be larger than 30%.
Aerosol particles suspended in a diluted gas with non-uniform temperature distribution are expected to experience a thermophoretic force. In theoretical treatment of thermophoresis, it is usually assumed that the particle temperature is equal to the surrounding gas temperature. However, this might not always be the case. In some particular applications, the particle temperature can significantly differ from the gas temperature. In the present paper, we theoretically investigate the effect of the particle temperature on the thermophoresis of nanoparticles in the free molecule regime. Theoretical formulas for the thermophoretic force and thermophoretic velocity are obtained based on the gas kinetic theory. As examples, a spherical Ag nanoparticle suspended in a dilute He gas is considered, and the Rudyak–Krasnolutski potential is employed to model the gas–particle interaction. It is found that the influence of the particle temperature on the thermophoresis of nanoparticles can be significant. With increasing particle size, the error due to the equal gas–particle temperature assumption can be neglected.