- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
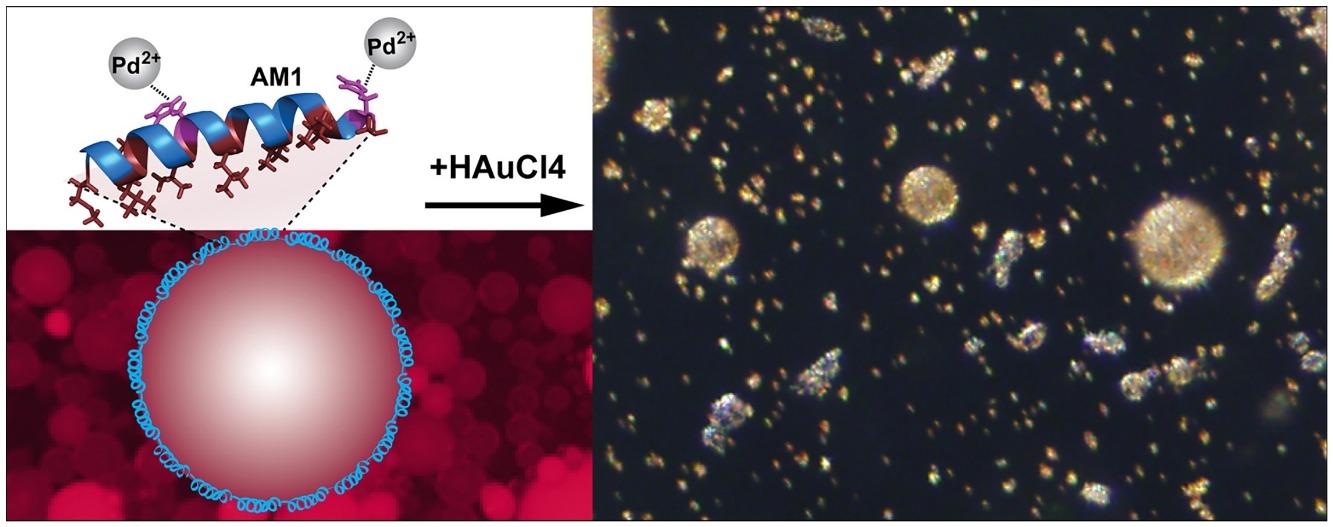
• Designer biosurfactant AM1 forms stable oil/water emulsions with Pd(II) ions.
• Pd(II)-AM1 interfacial network remains stable at acidic pH.
• Interaction of histidine residues with Pd(II) is by a different mechanism than Zn(II).
• Pd(II)-AM1 stabilised interface catalyses gold shell deposition around emulsions.
Designer biosurfactants can be used to stabilise and functionalise interfaces. One particularly promising use is the stabilisation of oil-in-water emulsions, enabling fine tuning physical, chemical and biological surface properties. The ability of emulsion systems to carry high payloads makes them attractive for applications in medicine, food and fragrances, and cosmetics. However, they have limited long-term stability. Here we sought to use the metal ion-chelating ability of the biosurfactant peptide, AM1, to precipitate the formation of a gold metal shell on AM1-stabilised emulsions by electroless plating. We found that replacing the commonly used zinc(II) with palladium(II) for coordination by histidine residues of adjacent AM1 peptides produced interfacial films that maintained elasticity at acidic pH. Proton NMR suggested a coordination mechanism independent of the imidazole ring of the histidines. Nevertheless. stabilisation of emulsions at low pH enabled the deposition of a gold shell, albeit by an unexpected mechanism. We propose that gold nanoparticles forming in bulk are adsorbed onto the peptide-stabilised interface, accumulating into a particulate coating. The resulting one-step method for nanoparticle precipitation and shell formation will be useful for the creation of biocompatible core–shell particles for applications where large payloads of hydrophobic active compounds require stability over long time periods.