- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
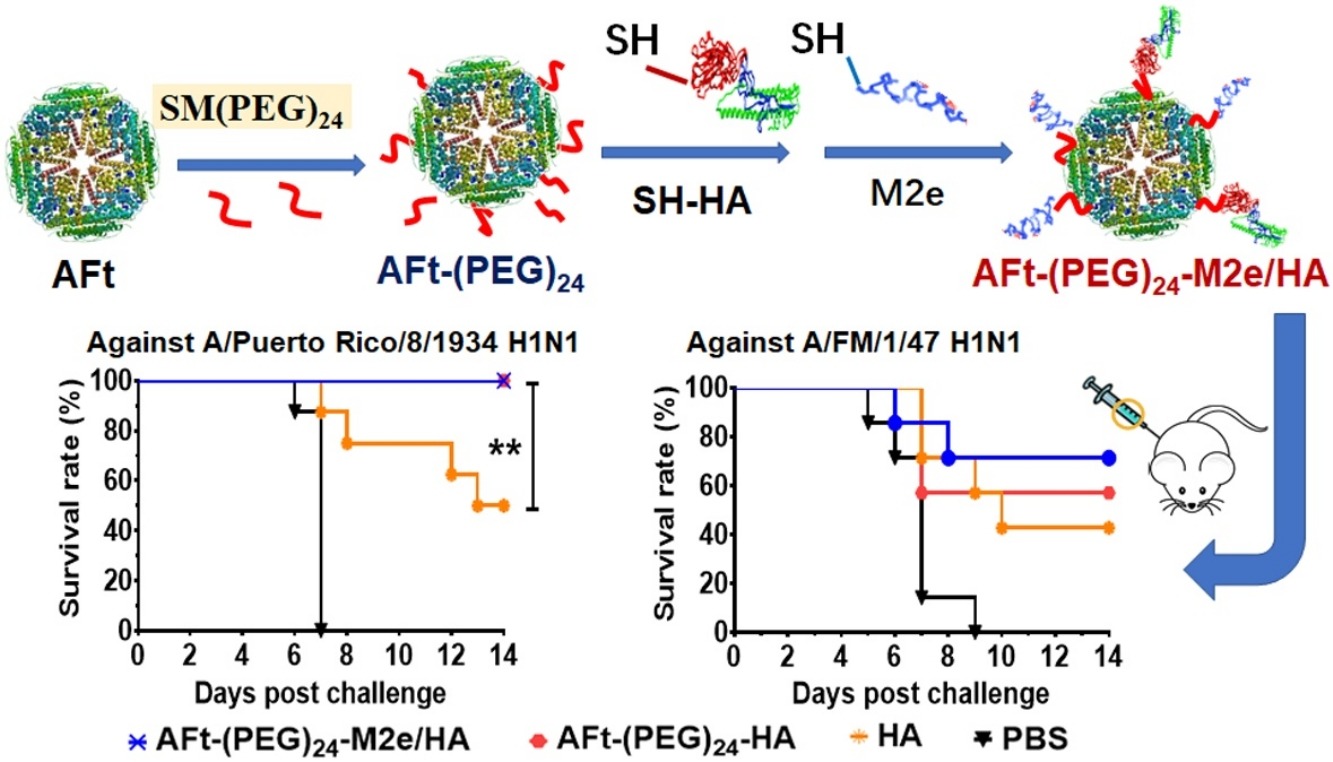
• M2e or/and HA antigen from H1N1 influenza virus was chemically conjugated to Aft.
• AFt-(PEG)24-HA can confer higher protective efficacy than AFt-(PEG)24-M2e antigen.
• AFt-(PEG)24-M2e/HA dual-antigen vaccine elicits M2e and HA specific antibodies.
• The dual-antigen vaccine provides complete protection against homologous H1N1 virus.
• The vaccine shows potential cross-protective effect against heterosubtypic virus.
Ferritin nanoparticles with self-assembling properties have been widely explored as vaccine carrier by displaying foreign antigens through genetic fusion strategy. In the present work, an apoferritin (AFt) nanoparticle was tested as influenza vaccine carrier by chemically conjugating a matrix protein 2 ectodomain (M2e) antigen peptide or/and the full-length hemagglutinin (HA) antigen on the outer surface of the AFt, with heterobifunctional sSMCC or SM(PEG)24 containing PEG chain as linkers. To each AFt nanoparticle, about 30–32 M2e or 1.8 HA antigen could be coupled. The AFt-(PEG)24-M2e, in which the M2e was coupled through SM(PEG)24 containing PEG chain, conferred higher protective efficacy in immunized mice than AFt-M2e did, but was less effective than AFt-(PEG)24-HA. When both M2e and HA were coupled, the synthesized dual-antigen vaccine candidate AFt-(PEG)24-M2e/HA elicited high level of M2e and HA antigen-specific antibodies and conferred 100% protection against lethal infection of homologous PR8 H1N1 virus strain and 70% protection against a heterologous A/FM/1/47 (FM1, H1N1) strain, which was more effective than the M2e or HA single antigen vaccine candidates. The potential cross-protective effect of the dual-antigen vaccine was further demonstrated by significant specific hemagglutination inhibition (HAI) titers in serum of the immunized mice against three other heterologous viral strains including A/Singapore/GP1908/2015 (IVR-180) H1N1, A/Anhui/1/2005 H5N1, and A/Hong Kong H3N2.