- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
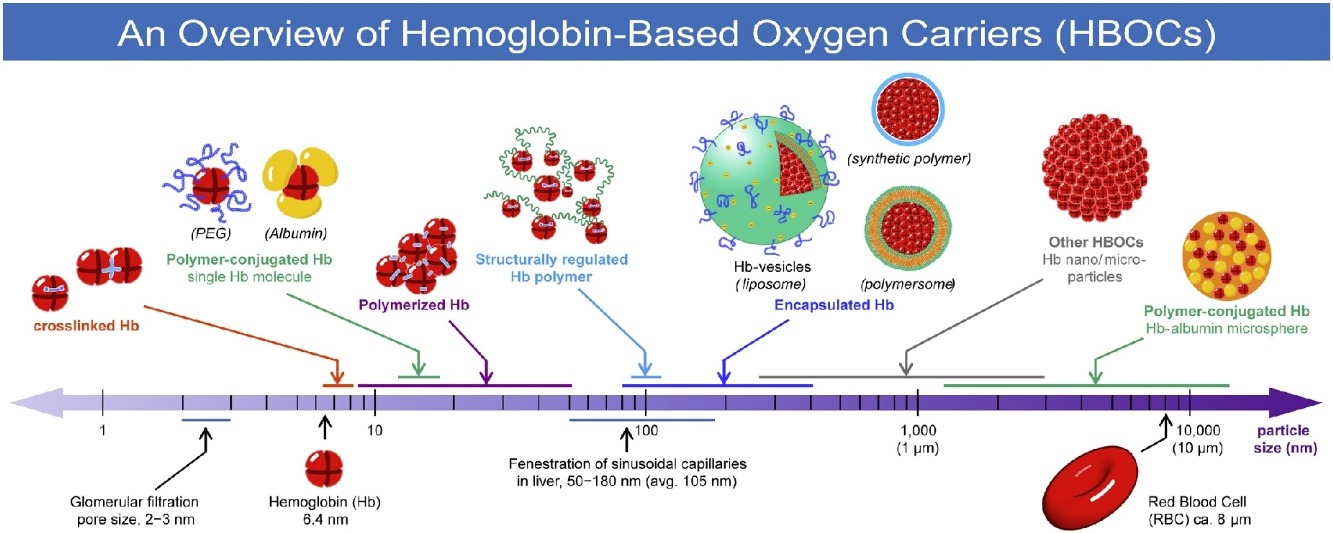
• Hb-based oxygen carriers (HBOCs) are studied to substitute for red blood cells.
• HBOCs include chemically-modified, genetically-altered, and encapsulated Hbs.
• Size control of HBOCs is a key to suppress side effects of Hb in blood circulation.
• Hb-vesicles (HbV) have a liposomal structure that resembles red blood cell membrane.
• Detailed preclinical studies of HbV clarified the in vivo safety and efficacy.
Blood transfusions are regarded as the most well-known and frequently performed cell transplantations. Although current transfusion systems are sophisticated, they cannot be freed from the inherent difficulties that include infection, short shelf life, and blood type mismatching. Artificial oxygen carriers produced using hemoglobin (Hb) are designated as Hb-based oxygen carriers (HBOCs), which are anticipated for use as biomaterials that have potential to resolve issues of transfusion by a radical paradigm shift. Various HBOCs, nanometer-sized to micrometer-sized bioparticles having an oxygen-carrying function, are developed for use as substitutes for red blood cells (RBCs). This paper presents an overview of the classification of HBOCs with reference to their histories, preparations, structures, functions, and in vitro and in vivo properties. Additionally, we give a more detailed introduction of our academic studies of liposome encapsulated Hb, designated as Hb-vesicles (HbV), which mimic the physiologically important corpuscular structure of RBCs. This review outlines perennial efforts and approaches to mimic RBC functions through chemical, genetic, and encapsulation techniques. It will provide important insights into the eventual realization of an alternative for RBC transfusion.