- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Phase transformation process of levofloxacin hydrochloride in two states.
• Thermodynamic and kinetic factors affecting phase transformation process.
• One mechanism for analyzing the formation and transformation process.
• Effective methods to control different solid forms.
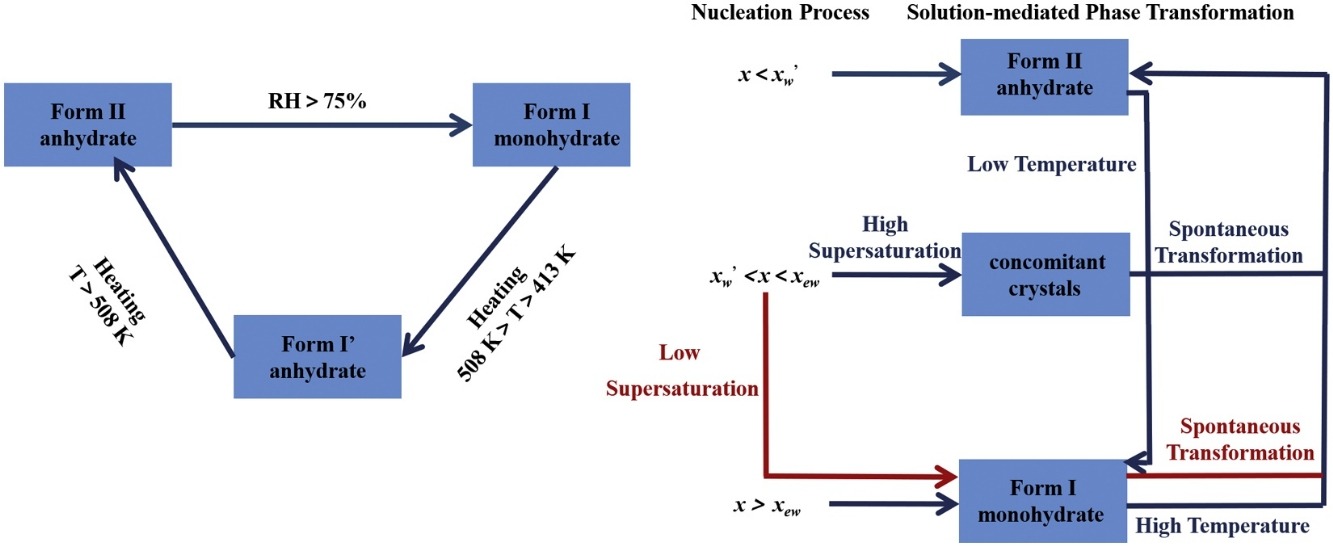
In this work, thermodynamic and kinetic factors that affect formation and phase transformation process of different solid forms of levofloxacin hydrochloride were investigated in detail. Dynamic vapor sorption experiments (DVS) and varying temperature-powder X-ray diffraction (VT-PXRD) experiments were carried out to study moisture-dependent stability, thermal stability as well as the transformation process between Form I and Form II. Critical water activities of levofloxacin hydrochloride were determined in temperature range of 5.0–45.0 °C. Raman spectroscopy was applied to in situ monitor the temperature-induced phase transformation and the hydration process of levofloxacin hydrochloride, and one possible mechanism consisting of multiple chemical equilibria was proposed to analyze the effect of thermodynamic and kinetic factors on the formation and transformation of different solid forms. Results show that the anhydrate, Form II would transform to the monohydrate, Form I more easily with the increasing water content. And the transition point moves toward higher water contents as the temperature increases. The results suggested that, except for thermodynamic factors, kinetic factors also play an essential role in controlling the solid forms of polymorphic compounds.