- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
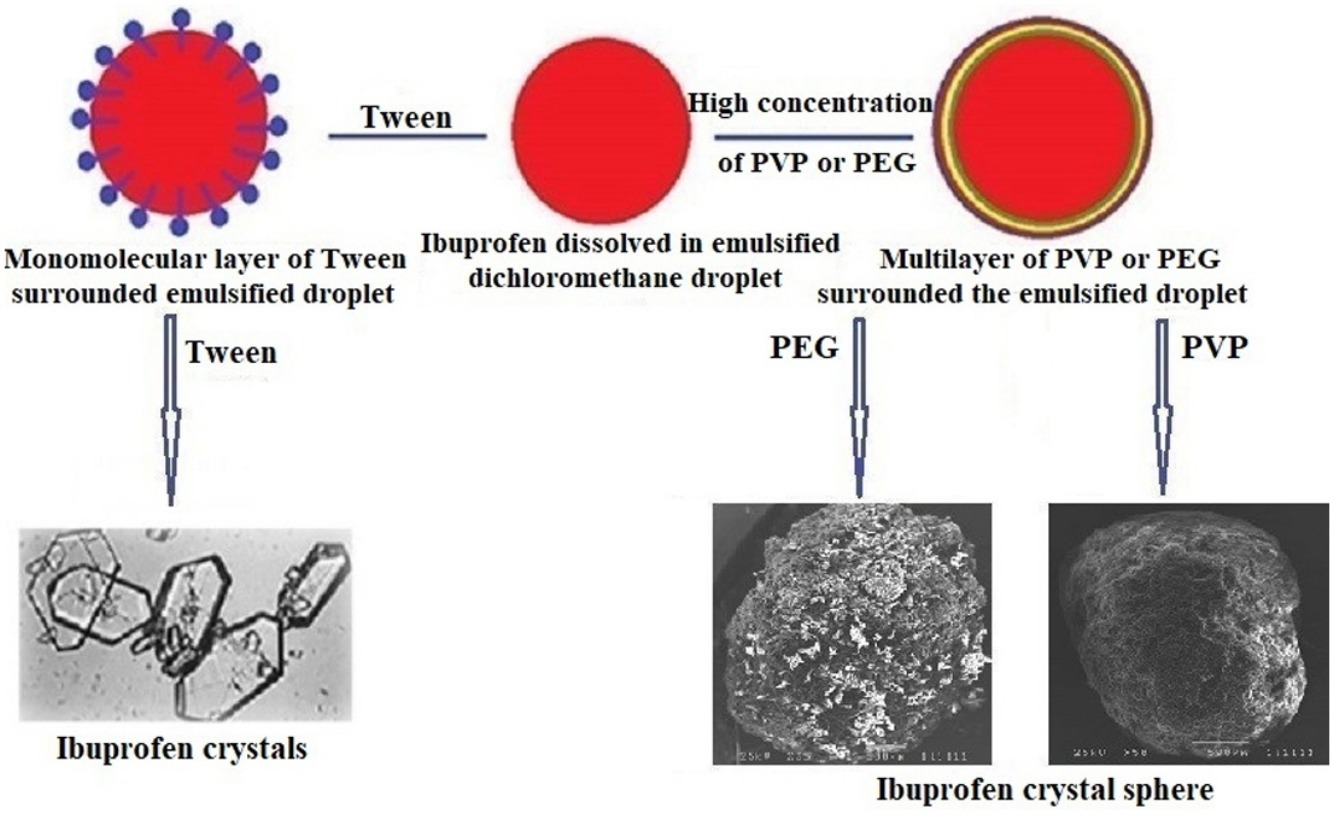
• Drug crystal spheres were prepared using drug encapsulation technique as a novel application.
• Ibuprofen was selected as a model drug.
• Hydrophilic colloid should be used at a high concentration.
• Low or high HLB surfactant did not produce drug crystal spheres.
An innovative application of the solvent evaporation technique was suggested. Solvent evaporation technique is a technique for drug encapsulation and nanosphere preparation. The widely used technique is also facing the problem of low actual drug entrapment percent, which is not economic from the industrial view. The goal of this work is trying to use the advantage of this technique concerning the product sphericity and the ability to control particle size, to prepare a drug as pure crystals spheres. Ibuprofen is selected as a model drug. The spheres are formed by using Polyvinyl pyrrolidone (PVP) or Polyethylene glycol (PEG) as an anti-aggregating agent but not formed on using tween or span. Particle size and actual drug content depend on the concentrations the anti-aggregating agent used. Surfaces of the drug crystal spheres are porous with empty sphere internal structure on using PVP but spongy and rough on using PEG. The drug has its identity chemical form in the drug crystal spheres. IR scan of spheres prepared on using PEG showed a characteristic ether peak. DSC showed melting endothermic peak of PEG, but X-ray showed minor change in the drug crystal patterns. Drug release profiles from crystal spheres prepared with the same anti-aggregating agent are close to each other. The drug release profiles from drug crystal spheres prepared by using PEG are more controlled than that prepared by using PVP. The drug release mechanism is diffusion. It was concluded that, the same technique could be suggested for preparation of other biomedical material in pure crystals spheres with controlled particle size. These properties may encourage to prepare very small particles with spherical shape for inhalation or injection as an innovative particle technology application for the widely used technique.