- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
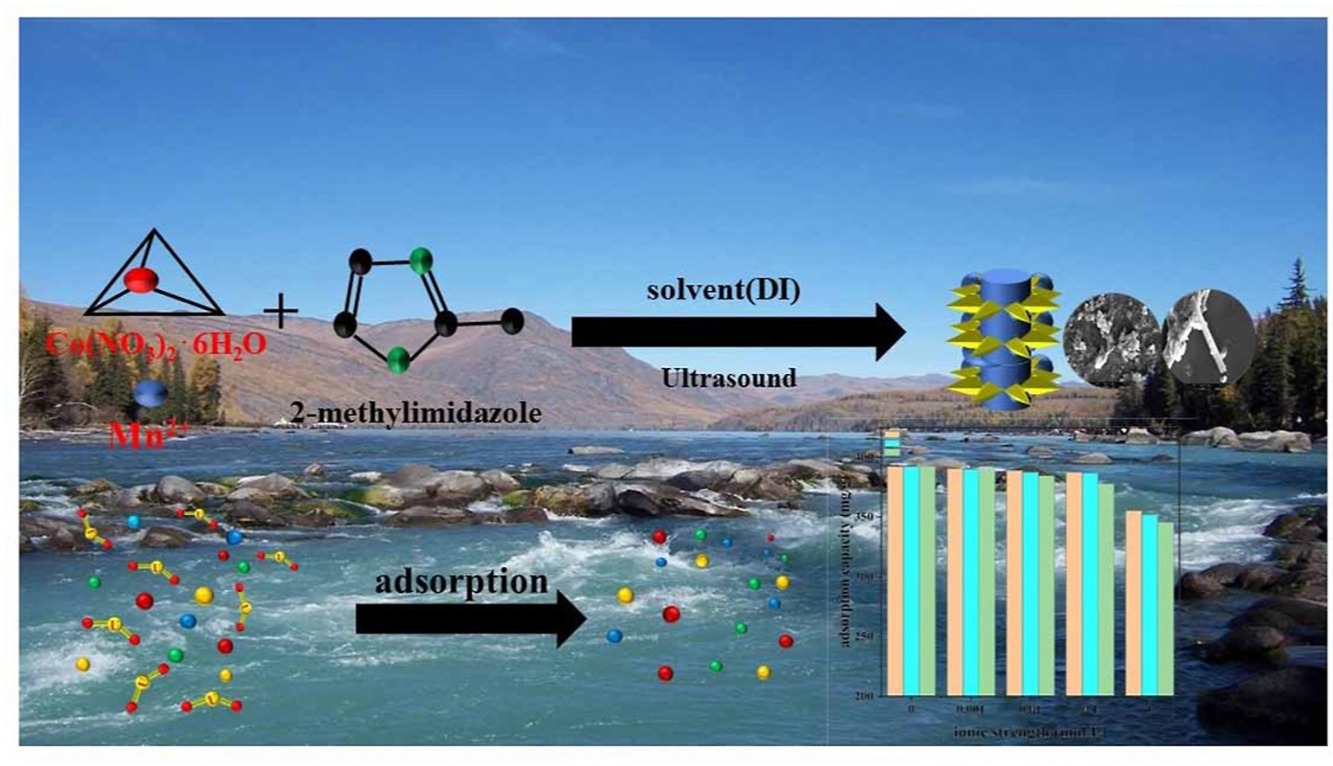
• Mn/Co-MOF was quickly synthesized in deionized water by ultrasound-assisted method and characterized successfully.
• Adsorption performance of U(VI) on Mn/Co-MOF was evaluated.
• Maximum adsorption capacity of Mn/Co-MOF for U(VI) in aqueous solution is 763.36 mg/g.
• Adsorption kinetics, thermodynamics and possible adsorption mechanisms of U(VI) are discussed.
Ultrasound-assisted synthesis of Mn/Co-MOF nanomaterial was used to capture uranium from aqueous solutions. Tests of Scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), Fourier transformed infrared spectra (FT-IR), Zeta potential analysis, thermogravimetric analysis (TGA), and X-ray diffraction (XRD) suggest that cobalt ions were replaced partially by manganese ions to generate MOF during the synthesis process and form manganous oxide particles loaded on the surface of Mn/Co-MOF. The optimal immobilization conditions of U(VI) were systematically studied by solution pH, kinetic, contact time and preparatory uranium concentration. XPS spectroscopy analysis indicated that the chelation of imidazole ring to uranium and Mn3O4 possibly played a certain role in the adsorption process. The results indicate that the adsorption isotherms of the Mn/Co-MOF for uranium suit Langmuir isotherm model (maximum adsorption capacity were 763.36 mg/g). Furthermore, the adsorption kinetics of Mn/Co-MOF match comfortably with the pseudo-second-order kinetic model.