- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
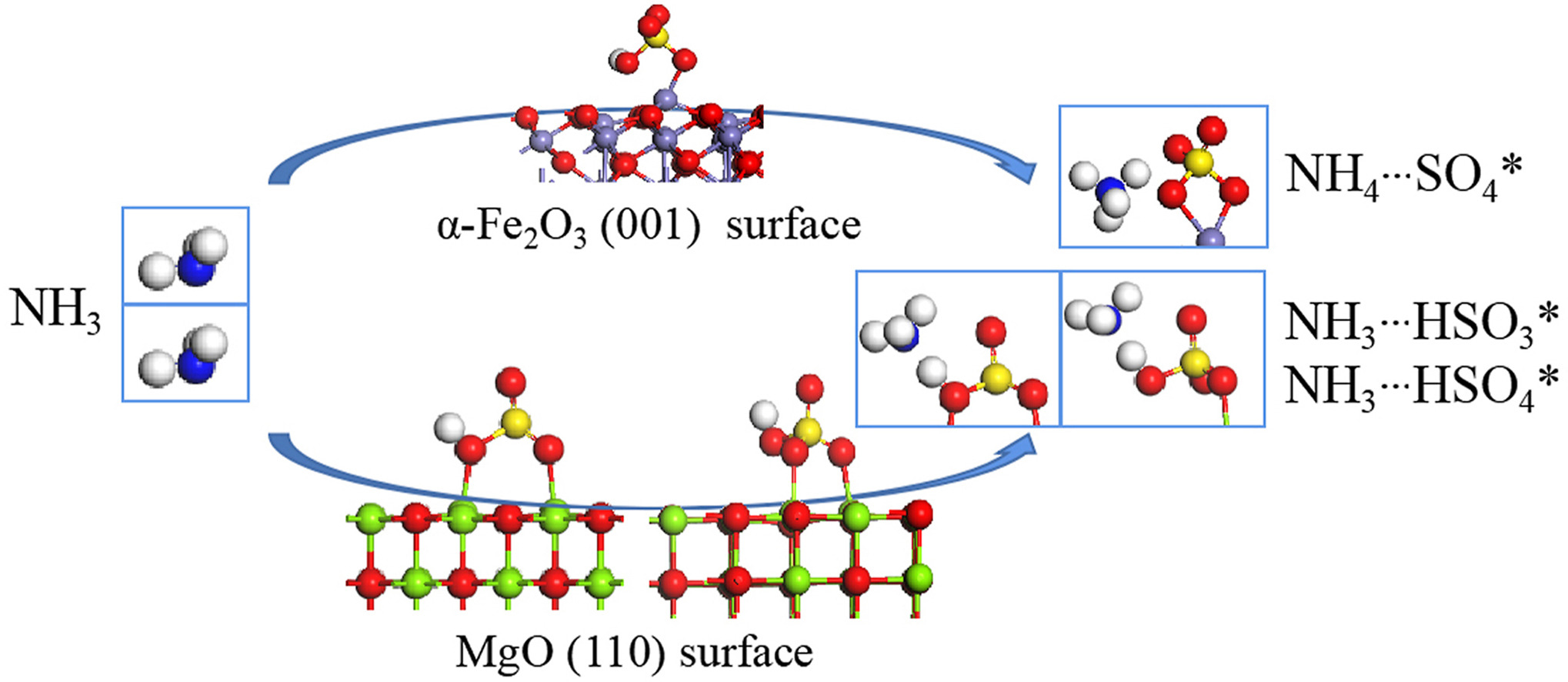
• Heterogeneous reactions of NH3 on mineral dust are explored by theoretical calculation.
• No hydrogen transfer reactions occur on the MgO surface.
• NH4+ formation occurs by hydrogen transfer from HSO4 sites on α-Fe2O3 (001) surface.
• Strong electron affinity of Fe3+ leads to great acidity of bisulfate on Fe2O3 surface.
Ammonium is an important atmospheric particulate component that dictates many environmental processes. The promotion of the heterogeneous conversion of NH3 to NH4+ by SO2 on different mineral dust surfaces displays remarkable discrepancies, especially on MgO and α-Fe2O3 surfaces, however, the underlying mechanisms are not well known. Here, using periodic density functional theory (DFT) calculation and Born-Oppenheimer molecular dynamics (BOMD) simulation, we explored the heterogeneous adsorption of NH3 on MgO (110) and α-Fe2O3 (001) surfaces in the presence and absence of SO2. The results show that on MgO (110) surface, hydrogen-bonding interactions of NH3 on both adsorbed hydroxyl or bisulfite/bisulfate sites are observed no matter whether SO2 is present or not. While, on the α-Fe2O3 (001) surface, significant conversion of NH3 to NH4+ occurs with the coexistence of SO2, which is due to the hydrogen transfer reaction from surface HSO4 to N in NH3. The fundamental reason may be that the stronger electron affinity of Fe3+ than Mg2+ results in adsorbed bisulfate and/or bisulfite with greater acidity on α-Fe2O3 surface than MgO surface. Our results give a molecular-level explanation for the heterogeneous conversion of NH3 to NH4+ on different mineral dust surfaces under complex air pollution conditions. Considering the fact that ammonium is abundant in secondary particulates, this work would help in understanding the rapid conversion of ammonia to ammonium and in developing classification governance policies for the key precursor pollutants in China.