- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
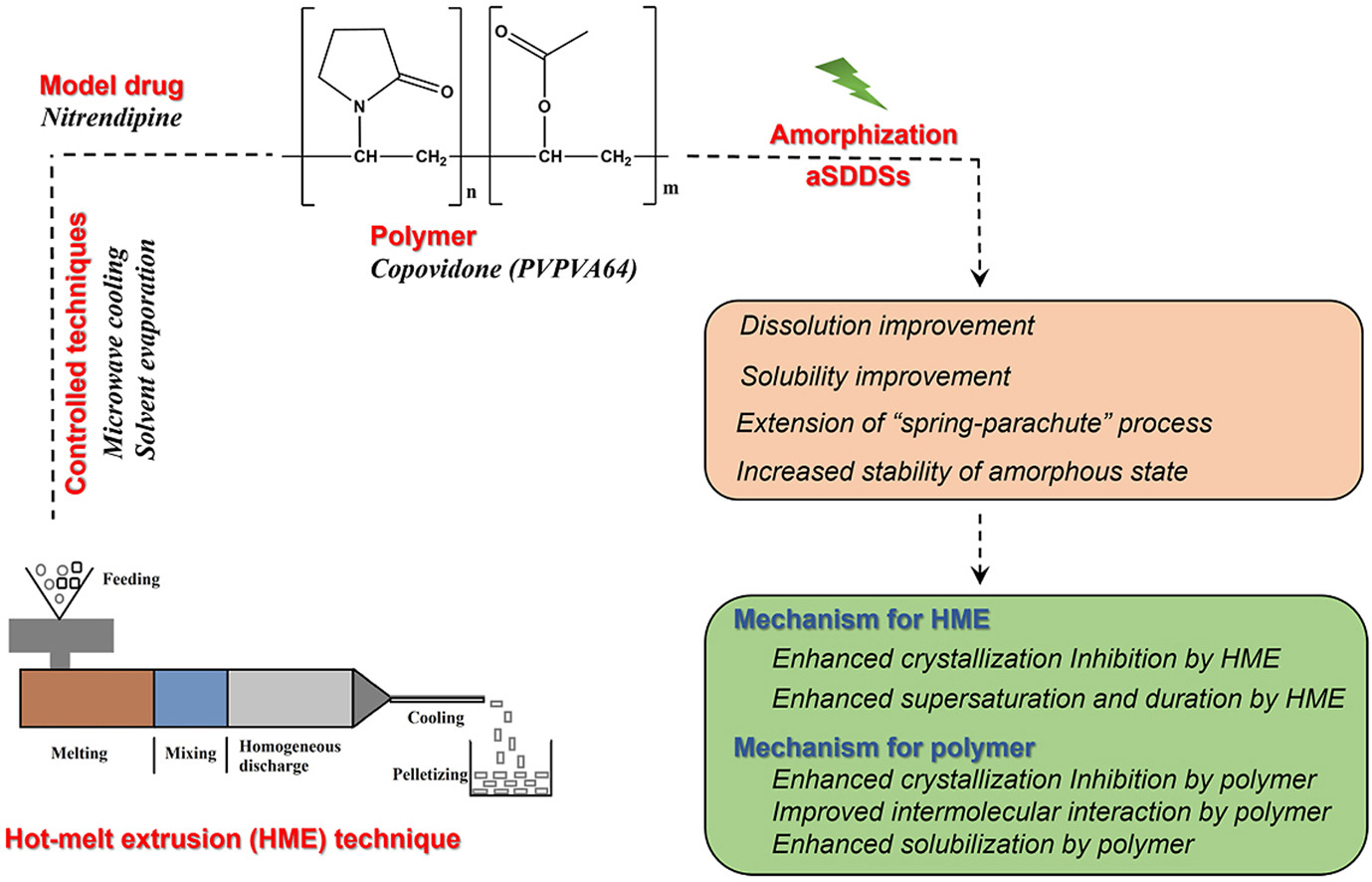
Despite the potential advantages of amorphism-induced supersaturation, the merit of new amorphization formation methods on the properties of the amorphous drug including the stability of the amorphous state, dissolution/solubility, supersaturation, and “spring-parachute” process is still poorly understood, particularly for certain amorphous supersaturating drug delivery systems (aSDDS). The present work aimed to explore the detailed merit of current attractive amorphization manufacturing methods (i. g., hot-melt extrusion (HME) technique) on the property improvement of aSDDS in form of amorphous solid dispersion microparticles by employing a model BCS II drug nitrendipine and a polyvinylpyrrolidone-based model polymer copovidone. Many aSDDS systems were developed by various methods, and their physicochemical properties were characterized by SEM, PXRD and DSC. HME-triggered amorphization induced superior supersaturation by the observation of the highest dissolution and solubility. HME induced the optimal supersaturation duration by the observed greatest extension of “spring-parachute” process (e. g., maximum AUCspring-parachute). HME technique is comparable with other techniques for the stabilization of amorphous state during storage. All aSDDS systems by HME and other methods showed improved long-term stability of the amorphous state in comparison to the pure amorphous drug. Fourier transformation infrared spectroscopy, Noyes-Whitney equation, nucleation theory and Gibbs free energy of transfer (ΔGt0) were used to analyze the underlying mechanisms. Molecular mechanism studies indicated that HME caused a stronger crystallization inhibition effect in the aSDDS systems than other methods, but molecular interaction is not a dominant mechanism for property enhancement caused by HME. For the mechanism associated with the polymer itself (PVPVA64), it could inhibit the drug recrystallization, solubilize the drug spontaneously and cause the improved molecular interactions in all aSDDS systems. This study provided a deep insight into detailed advantage of HME-triggered supersaturation/amorphization and facilitated the applications of the technique both in the field of particuology and in pharmaceutical industry.