- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Increased calcium content in oxygen carrier enhances the immobilization of selenium.
• As the number of cycles increases Ca2Fe2O5 is more stable for retaining selenium.
• MgO and CaO in bottom ash have obvious fixing effect on selenium.
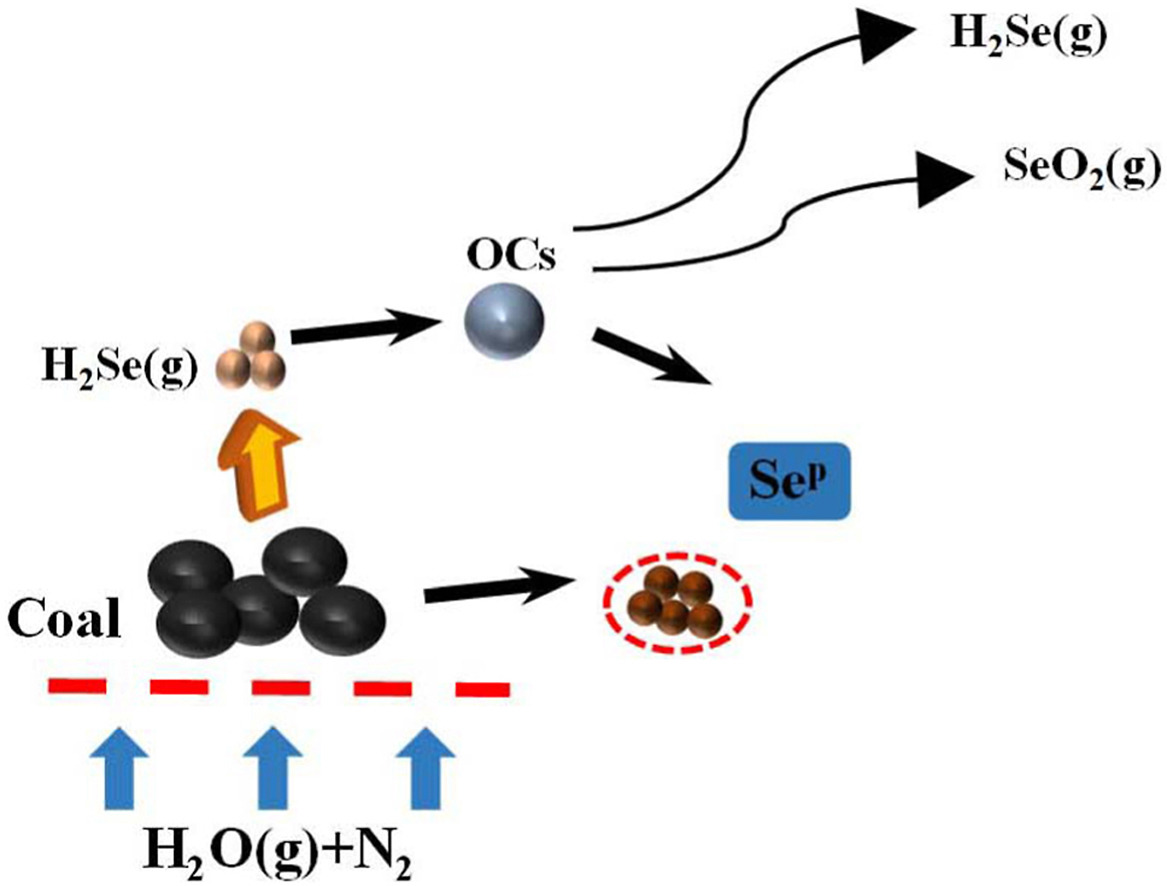
Selenium pollution by coal utilization is of increasing concern. Calcium-iron (Ca–Fe) oxygen carriers (OCs) and alkali metal ions have strong inhibitory effects on selenium, which can reduce the emissions of selenium vapor. The retention mechanisms of selenium by Fe2O3, CaFe2O4, Ca2Fe2O5 and bottom ash are investigated during chemical looping gasification (CLG). Iron-based OC can oxidize H2Se(g) to SeO2(g); furthermore, lattice oxygen is released by Fe2O3, contributing to the formation of an Fe–O–Se structure to retain selenium and form selenite. Because calcium ferrite is poorly oxidizing, it cannot oxidize H2Se(g), but the CaO produced when OCs are reduced can react with H2Se(g) to form CaSe(s), and this process can be promoted by H2S(g). The best retention rates reached 32.301% when Ca2Fe2O5 was used. In the cyclic experiment, the selenium retention of the bottom ash gradually increases. Alkali metal ions in bottom ash are the main factor in retaining selenium. Ca2+ and Mg2+ do not easily vaporize due to their high melting points; therefore, their selenium retention is significantly better than that of K+ and Na+. This research provided a new idea for the removal of selenium by using OCs and bottom ash particles during CLG.