- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
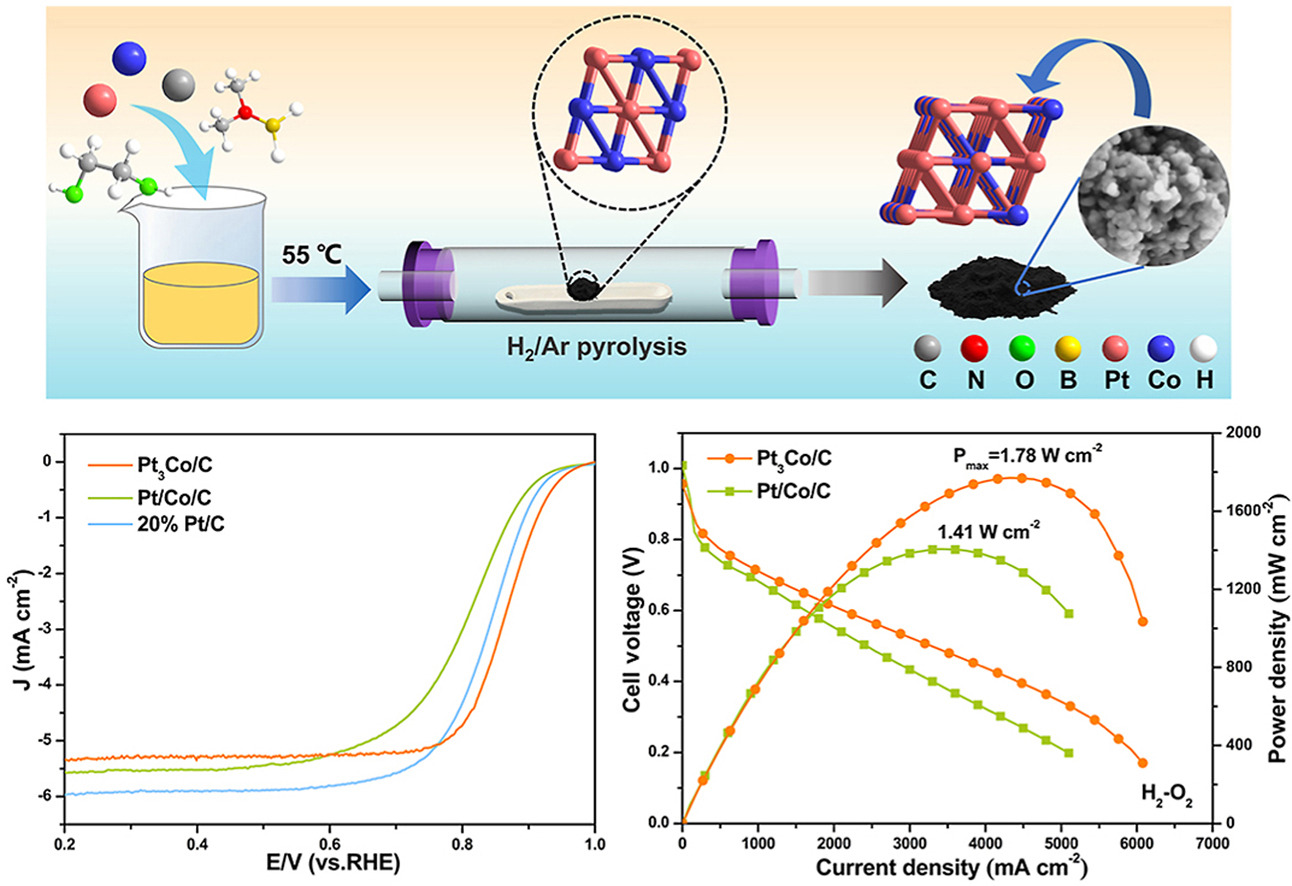
• Highly ordered Pt3Co nanoparticles were prepared by a two-step reduction strategy.
• Pt3Co/C has advantages of high reactivity, fast mass transfer and lower resistance.
• The half wave potential of Pt3Co/C reached 0.87 V, showing excellent ORR performance.
• The mass activity reached 0.67 A mgpt−1, exceeding the DOE standard (0.44 A mgpt−1).
Based on the volcanic relationship between catalytic activity and key adsorption energies, Pt–Co alloy materials have been widely studied as cathode oxygen reduction reaction (ORR) catalysts in proton exchange membrane fuel cells (PEMFCs) due to their higher active surface area and adjustable D-band energy levels compared to Pt/C. However, how to balance the alloying degree and ORR performance of Pt–Co catalyst remains a great challenge. Herein, we first synthesized a well-dispersed Pt/Co/C precursor by using a mild dimethylamine borane (DMAB) as the reducing agent. The precursor was calcined at high temperature under H2/Ar mixed gas by a secondary reduction strategy to obtain an ordered Pt3Co intermetallic compound nanoparticle catalyst with a high degree of alloying. The optimization of electronic structure due to Pt–Co alloying and the strong metal-carrier interaction ensure the high kinetic activity of the cell membrane electrode. Additionally, the high degree of graphitization increases the electrical conductivity during the reaction. As a result, the activity and stability of the catalyst were significantly improved, with a half-wave potential as high as 0.87 V, which decreased by only 20 mV after 10000 potential cycles. Single-cell tests further validate the high intrinsic activity of the ordered Pt3Co catalyst with mass activity up to 0.67 A mgpt−1, exceeding the United States Department of Energy (US DOE) standard (0.44 A mgpt−1), and a rated power of 5.93 W mgpt−1.