- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
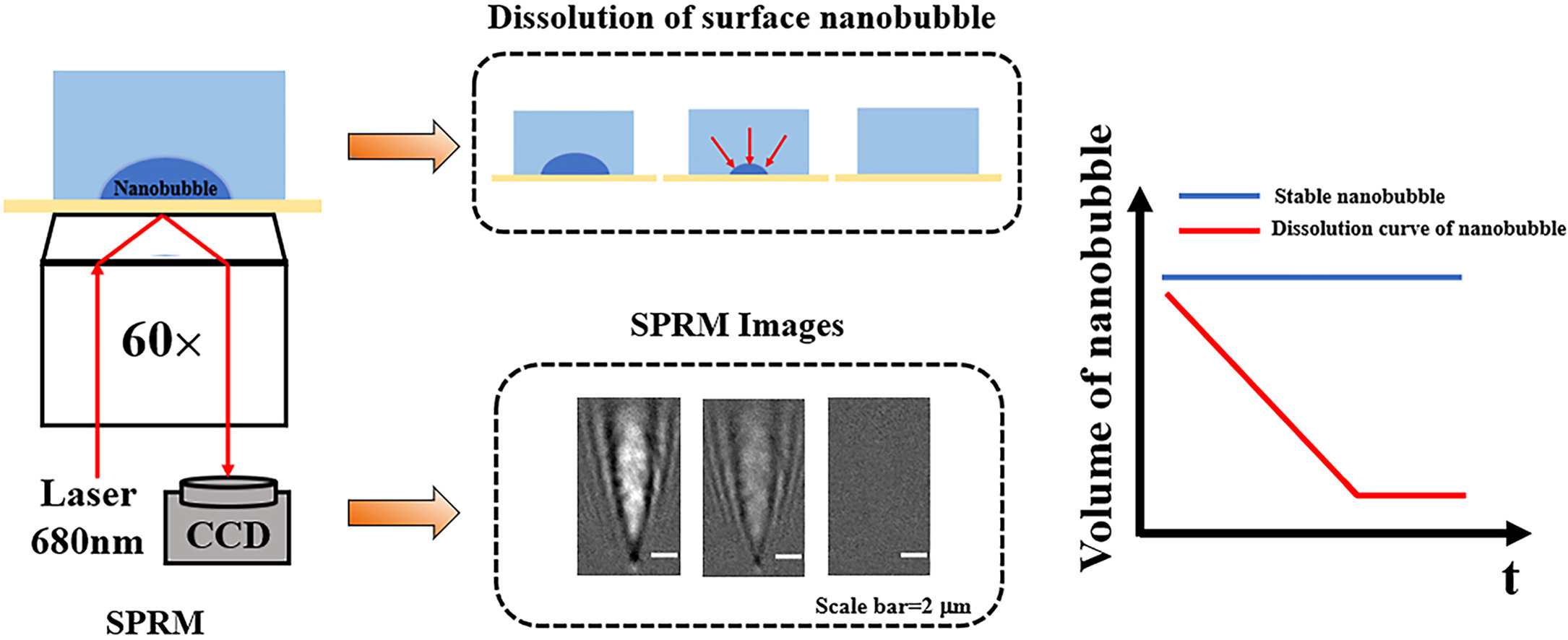
• Dissolution rates of surface nanobubbles are measured by surface plasmon resonance microscopy.
• High concentration electrolyte solutions promote the dissolution of surface nanobubbles.
• Roles of electrostatic interaction on the stability of surface nanobubbles are explored.
Surface nanobubbles are spontaneously formed at the interface between hydrophobic surfaces and aqueous solutions, which show extraordinarily longer lifetime than that was predicted by the classical thermodynamics model. In the present work, by using a surface plasmon resonance microscopy (SPRM) to quantitatively measure the dissolution kinetics of individual surface nanobubbles in real time, we explored the effects of ionic strength and pH value on the dissolution rates (lifetime) of nanobubbles. The results revealed that nanobubbles could exist stably for a long time in low-concentration electrolyte solutions or high-concentration non-electrolyte solutions, while they dissolved quickly in high-concentration electrolyte solutions. With the increase of ionic strength, the dissolution rates were accelerated by 2–3 orders of magnitude, and thus the lifespan of these surface nanobubbles was significantly shortened. In addition to ionic strength, it was further found that, with the increase of acidity or alkalinity of the solution, the dissolution rates of the surface nanobubbles were faster than that in neutral solution. These results demonstrated that the interfacial charge enrichment significantly contributed to the extraordinary stability of the surface nanobubbles.