- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
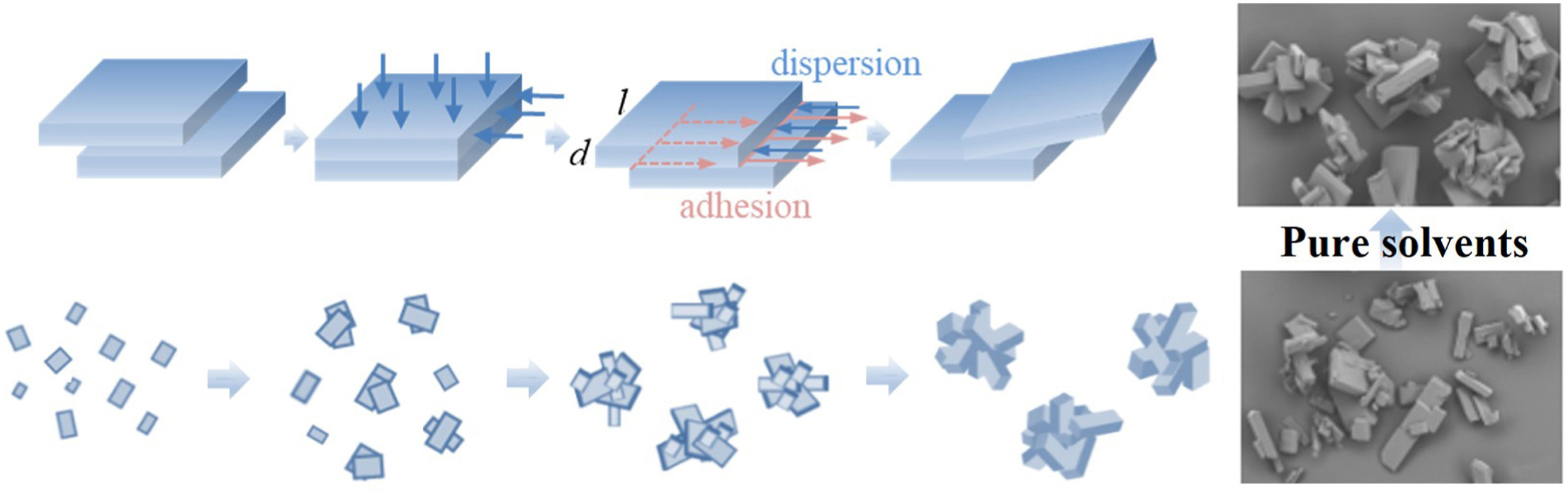
• Obtain agglomerates of platy aspirin by cooling crystallization in pure solvents.
• Determine suitable solvents and the maximum stirring rate for agglomeration.
• Elucidate a feasible mechanism for the agglomeration process of platy crystals.
• Study the effect of stirring rate, cooling rate, and supersaturation on agglomeration.
Agglomeration with improved flowability for platy crystals is desirable in pharmaceutical downstream processing. The formation of agglomerates in pure solvents without the aid of bridging liquids is a convenient and low-cost method compared with complex spherical crystallization. In this work, the adhesion free energies between aspirin crystals in six solvents were calculated using Lifshitz-van der Waals acid-base theory to screen suitable solvents for agglomeration. The maximum stirring rate for agglomeration was determined by adhesion forces and dispersion forces. Then the agglomerates of plate-shaped aspirin were successfully prepared in acetone, methanol, ethanol, 2-propanol, and ethylene glycol without additives by simple cooling crystallization. The interactions between solvent and crystal surfaces were also used to explain the outcomes. A feasible mechanism for the agglomeration process of platy crystals was elucidated, involving the adhesion of dominant crystal facets at the beginning. The effect of stirring rate, cooling rate, and initial supersaturation on agglomeration degree and particle size of aspirin agglomerates were studied. The obtained aspirin agglomerates under the optimal conditions exhibited a uniform particle size distribution, a high agglomeration degree, and superior flowability.