- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
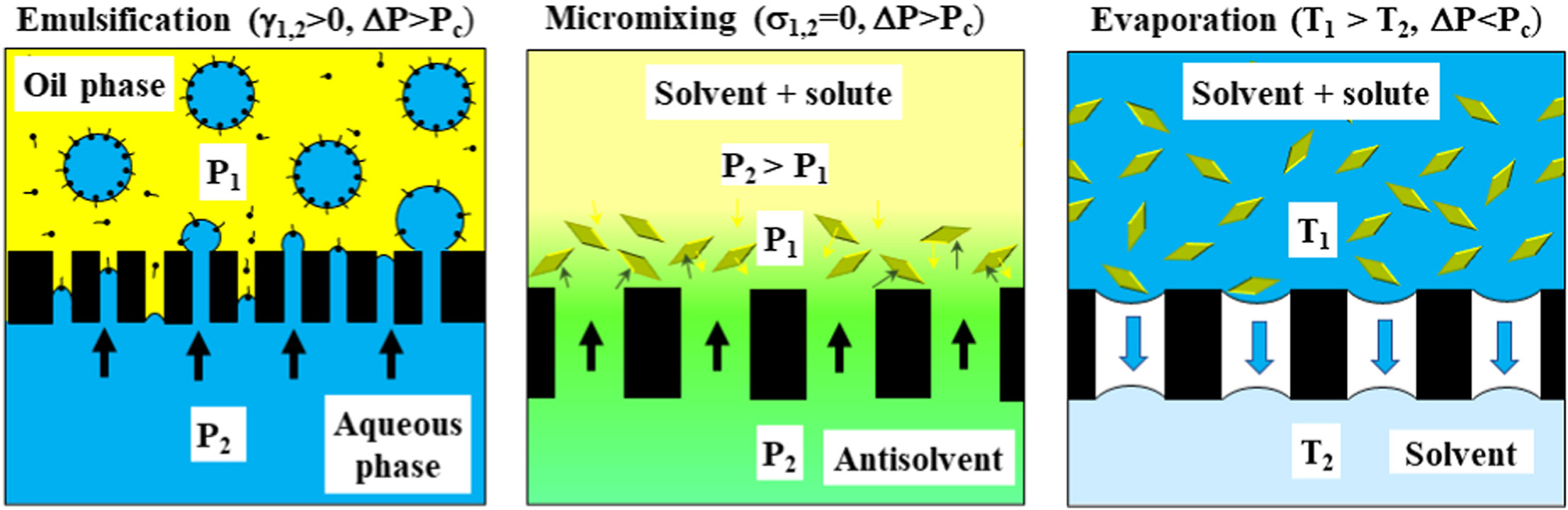
• Particles can be generated by dispersion, mixing or evaporation through membrane.
• In evaporation processes, membrane allows gas diffusion but prevents convective flow.
• In mixing processes, transmembrane pressure overcomes capillary pressure in pores.
• Solvent/antisolvent mixing via membrane leads to creation of supersaturation.
• Supersaturation is relieved through crystal nucleation or flash nanoprecipitation.
Synthetic microporous membranes are increasingly used for energy-efficient and controlled production of micro- and nanoparticles and micro- and nanoemulsions with tuneable morphology and physicochemical properties through various micromixing, emulsification, and evaporation processes. In emulsification processes, the membrane pores are used for dispersed phase injection and size-controlled generation of droplets and droplet-templated particles. In micromixing processes, membrane is utilised as a micromixer for mixing two miscible liquids, usually solvent and antisolvent-rich solutions, which leads to the creation of supersaturation and subsequent nanoprecipitation or crystallisation. In membrane evaporation processes, membrane is used to prevent phase dispersion while allowing efficient molecular diffusion of solvent and/or antisolvent vapour through gas-filled pores. Membrane dispersion processes can be operated continuously by decoupling shear stress on the membrane surface from cross flow using tube insets, flow pulsations, swirling flow, membrane oscillations or membrane rotations. Droplet generation and solidification can be performed continuously in a single pass by connecting membrane module with a downstream reactor. Membrane dispersion processes can be used for production of nanoparticles such as nanovesicles (liposomes, micelles, ethosomes, and niosomes), nanogels, polymeric, lipid and metallic nanoparticles, and nanocrystals. The main advantages of membrane-assisted particle generation are in low energy consumption, controlled geometry and hydrodynamic conditions at the microscale level, flexible throughput due to modular and scalable design of membrane devices, and a wide choice of available microporous membranes with various wall porosities, wettabilities, pore sizes, and pore morphologies to suit different applications.