- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
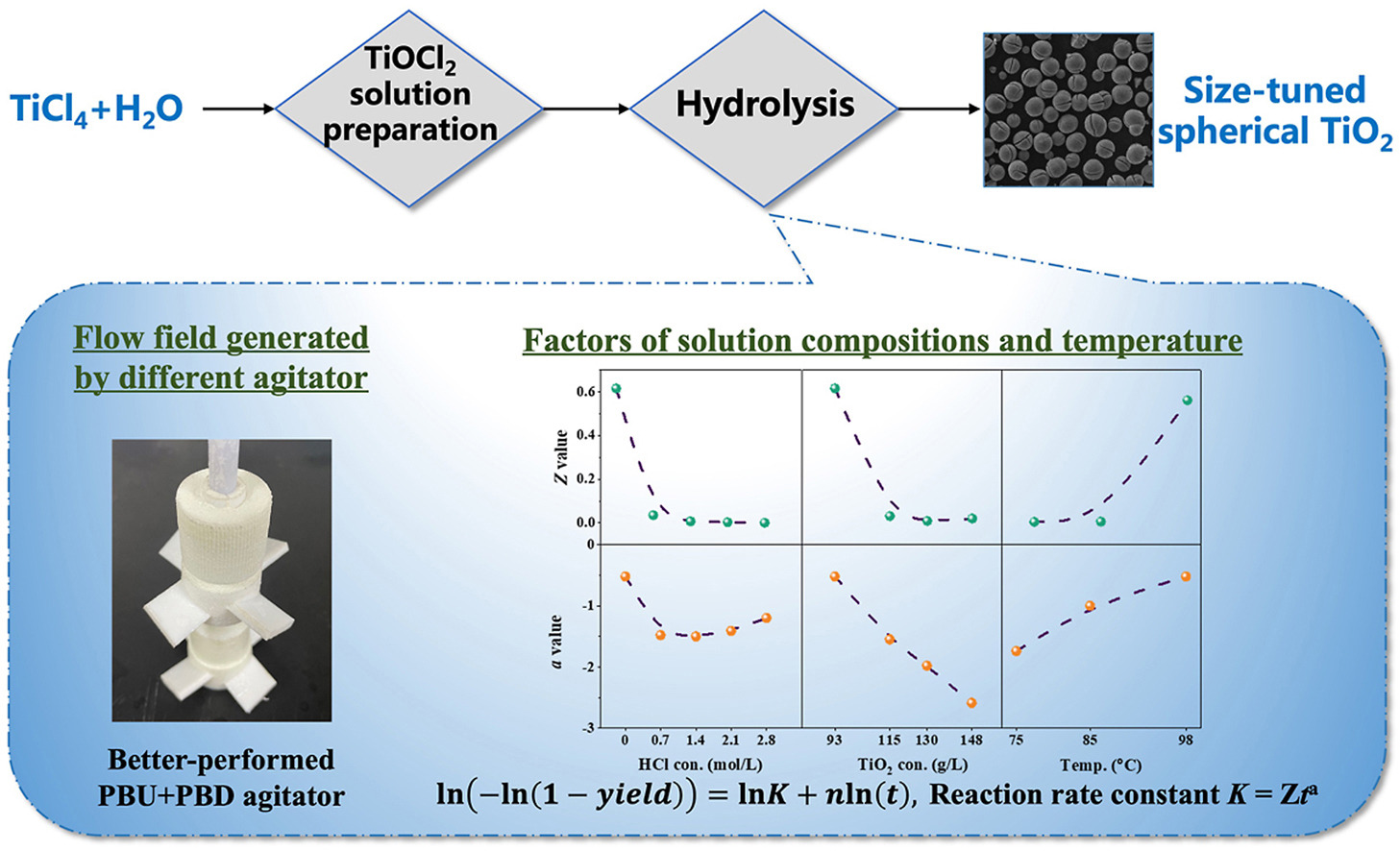
• Flow field, solution compositions, and temperature are key factors for hydrolysis.
• Morphology and powder size of TiO2 via hydrolyzing TiCl4 solution can be tuned.
• Cheng and Wunderlich modified Avrami equation are used for the kinetic fitting.
• Reaction rate constant and active nucleus deactivation rate index are involved.
Hydrolysis of TiCl4 solution is capable of preparing microscale TiO2 particles. This research studied the synthesis of microscale spherical TiO2 powders and the hydrolysis kinetics. The effects of the flow field generated by different agitators and baffles in the crystallizer, the initial free acid concentration, the initial equivalent TiO2 concentration, and the temperature on the hydrolysis progress and powder morphology were systematically studied. The results show that the flow field in a crystallizer can significantly affect the morphology and particle size of the powders, and the axial flow can improve the sphericity of the powders. The increased free HCl and equivalent TiO2 concentrations in the pregnant solution inhibit the forward hydrolysis reaction, prolong the time to reach equilibrium, and reduce the yield. An appropriate temperature matching the compositions of the pregnant solution is crucial for the powder morphology and size. Powders with sizes ranging from around 5 μm–40 μm can be tuned under controlled flow field, solution compositions, and temperature conditions. In addition, the Cheng and Wunderlich modified Avrami equation was used for the crystallization kinetic modeling. The effects of the free HCl concentration, equivalent TiO2 concentration, and hydrolysis temperature are reflected in the reaction rate constant and active nuclei reduction index. Increasing the free HCl and equivalent TiO2 concentrations will reduce the reaction rate constant and accelerate the deactivation of the active nuclei, thus increasing the final powder size, while increasing the temperature will lead to the opposite results.