- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
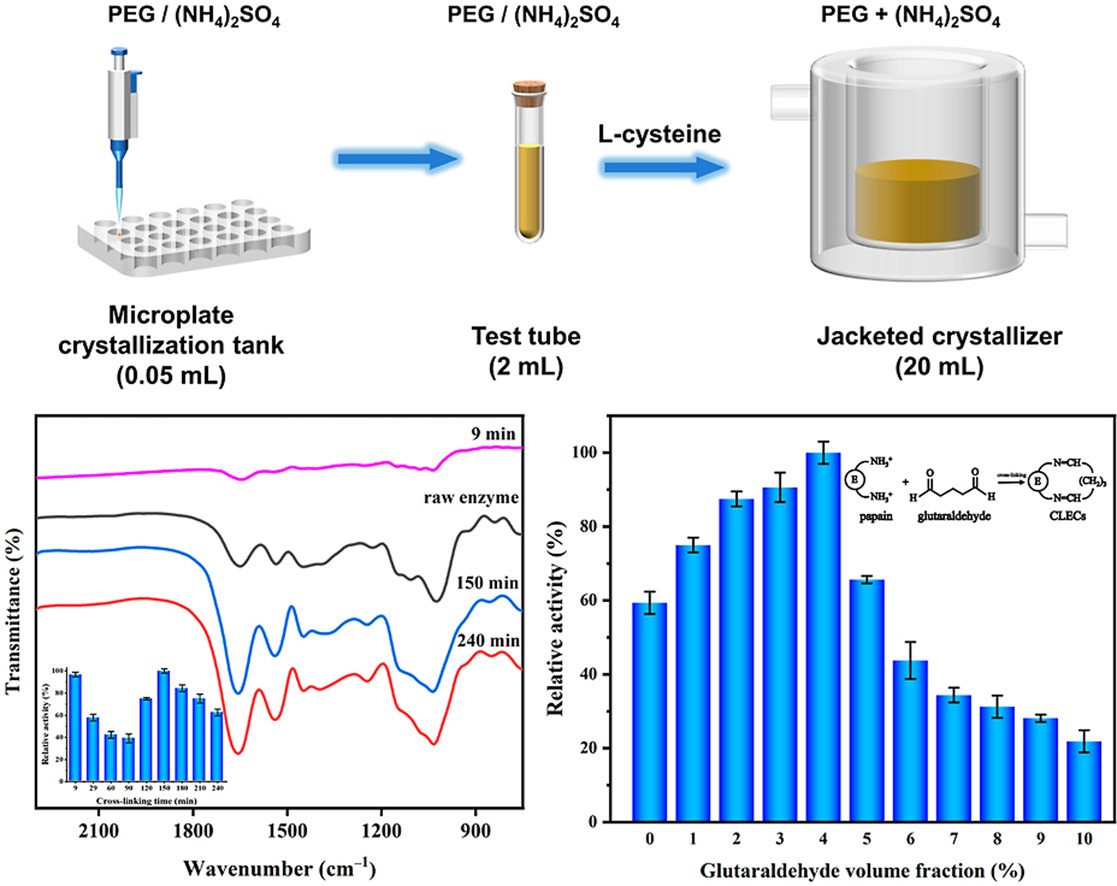
• Papain crystals was obtained by the synergistic precipitate.
• l-cysteine increased the enzyme activity by affecting the conformation of papain.
• Three types of forces facilitated the binding between papain and l-cysteine.
• Papain CLECs were successful prepared with higher stability and wider application.
In this study, we prepared cross-linked enzyme crystals (CLECs) of papain to further broaden the application of the enzyme with high activity in extreme environments. Initially, papain crystals were successfully obtained based on the micro-batch, batch, and expanded batch crystallization experiments. Specifically, ammonium sulfate and polyethylene glycol 6000 (PEG6000) were synergistically used as the precipitants, while l-cysteine was applied to enhance the activity of papain. Furthermore, the interaction between l-cysteine and papain was modeled by molecular docking technique. It was found that l-cysteine could form a hydrogen bond with aspartic acid residue (Asp) at site 158, and the electrostatic attraction with lysine residue (Lys) at site 156 was also quite obvious. Then the enzyme crystals were cross-linked by glutaraldehyde at optimized conditions. The papain CLECs were identified by various methods, and it was found that the thermal stability and enzymatic activity both increased compared to the raw enzyme. More importantly, it could be applied at more rigorous conditions, for example, pH of 4.