- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
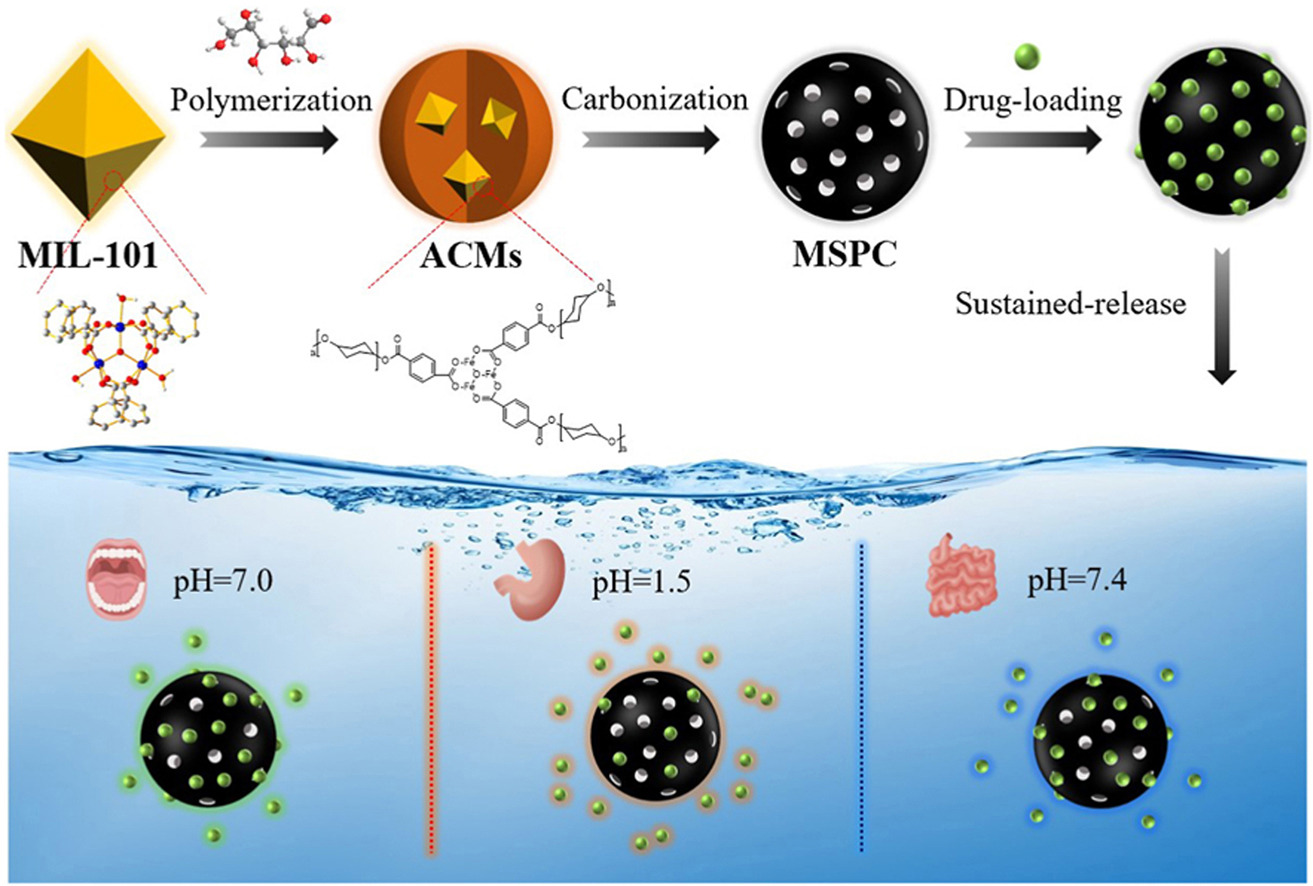
• A new strategy to prepare MIL-101(Fe)-derived spherical porous carbon.
• MSPC exhibits an excellent sustained-release performance toward nitroimidazole drugs.
• A new perspective for preparing mesoporous carbon spheres as drugs carrier.
Metal-organic framework (MOF) with a buildable internal structure has aroused great interest focus as self-sacrificing precursors of porous carbon (PC). However, as a drug carrier, the MOF-derived PC developed thus far are generally composed of irregular powder shape due to their crystalline nature, which consequently causing the cerebral infarction, cerebral thrombosis, and other blood diseases. In this article, we propose a novel approach to constructing amorphous carbon microspheres (ACMs) by distorting the topological network through hydrothermal treatment precursors of MIL-101(Fe). Then, a distinctive MIL-101(Fe)-derived spherical porous carbons (MSPC) is achieved through high temperature calcination toward ACMs. Effects of the glucose initial concentration and hydrothermal treatment time on the sphericity of the as-prepared mesoporous MSPC were investigated in depth. And the loading capacities and sustained-release performances of nitroimidazole drugs over MSPC through simulation internal environment of human body at different pH values was systematically evaluated. The nitroimidazole drugs loading rate and release time of MSPC are 10% and 17 h under preferred process. Furthermore, the MSPC exhibited very low toxicity on Hela cells and 293T cells at the concentrations tested (10–800 μg mL−1). This study, therefore, supports the potential of the mesoporous carbon spheres as a carrier for nitroimidazole drug delivery.