- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
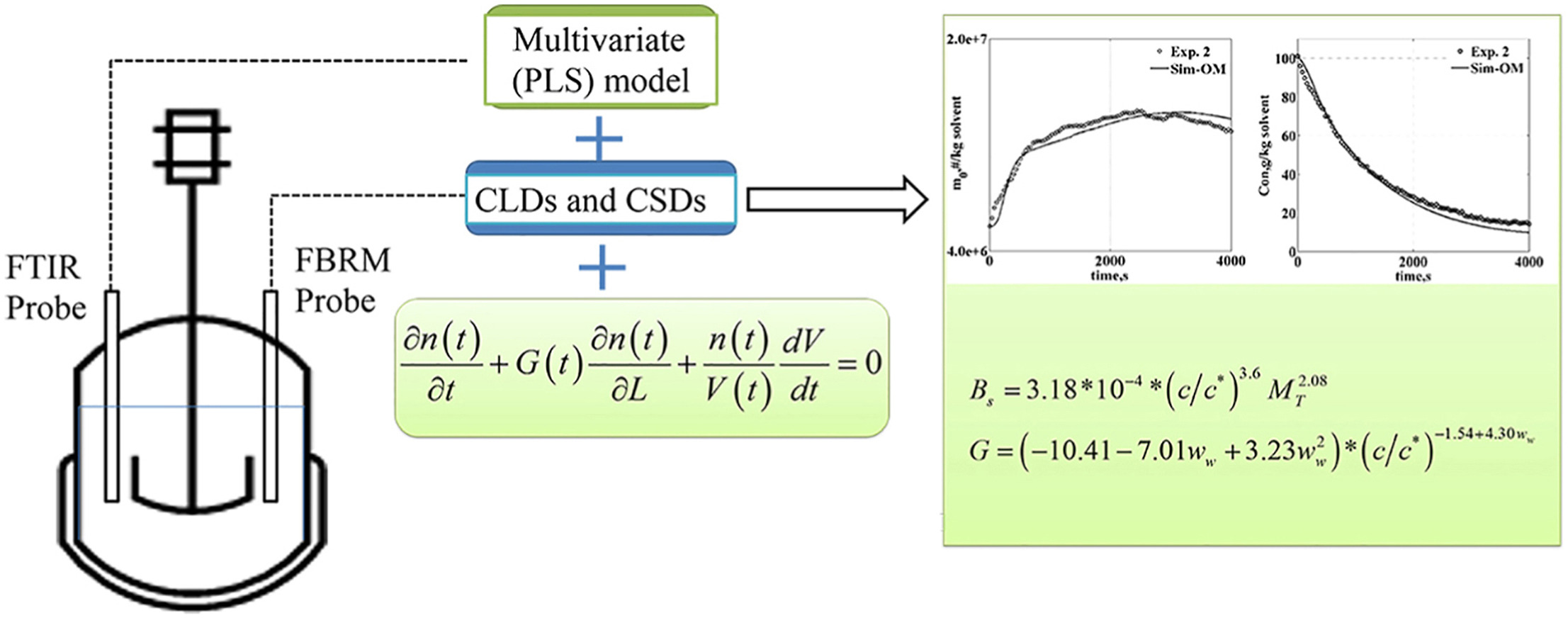
• Antisolvent crystallization of benzoic acid in semi batch crystallizer.
• Population balance models based on different supersaturation expressions are considered.
• Secondary nucleation and growth kinetics incorporating solvent composition are estimated.
• The identified secondary and growth kinetics of benzoic acid is validated experimentally.
The nucleation and growth kinetics of benzoic acid were determined in a population balance model, describing the seeded batch antisolvent crystallization process. The process analytical technologies (PATs) were utilized to record the evolution of chord length distributions (CLDs) in solid phase together with the concentration decay in liquid phase, which provided essential experimental information for parameter estimation. The model was solved using standard method of moments based on the moments calculated from CLDs and solute concentration. A developed model, incorporating the nucleation and crystal growth as functions of both supersaturation and solvent composition, has been constructed by fitting the zeroth moment of particles and concentration trends. The determined kinetic parameters were consequently validated against a new experiment with a different flow rate, indicating that the developed model predicted crystallization process reasonably well. This work illustrates the strategy in construct a population balance model for further simulation, model-based optimization and control studies of benzoic acid in antisolvent crystallization.