- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
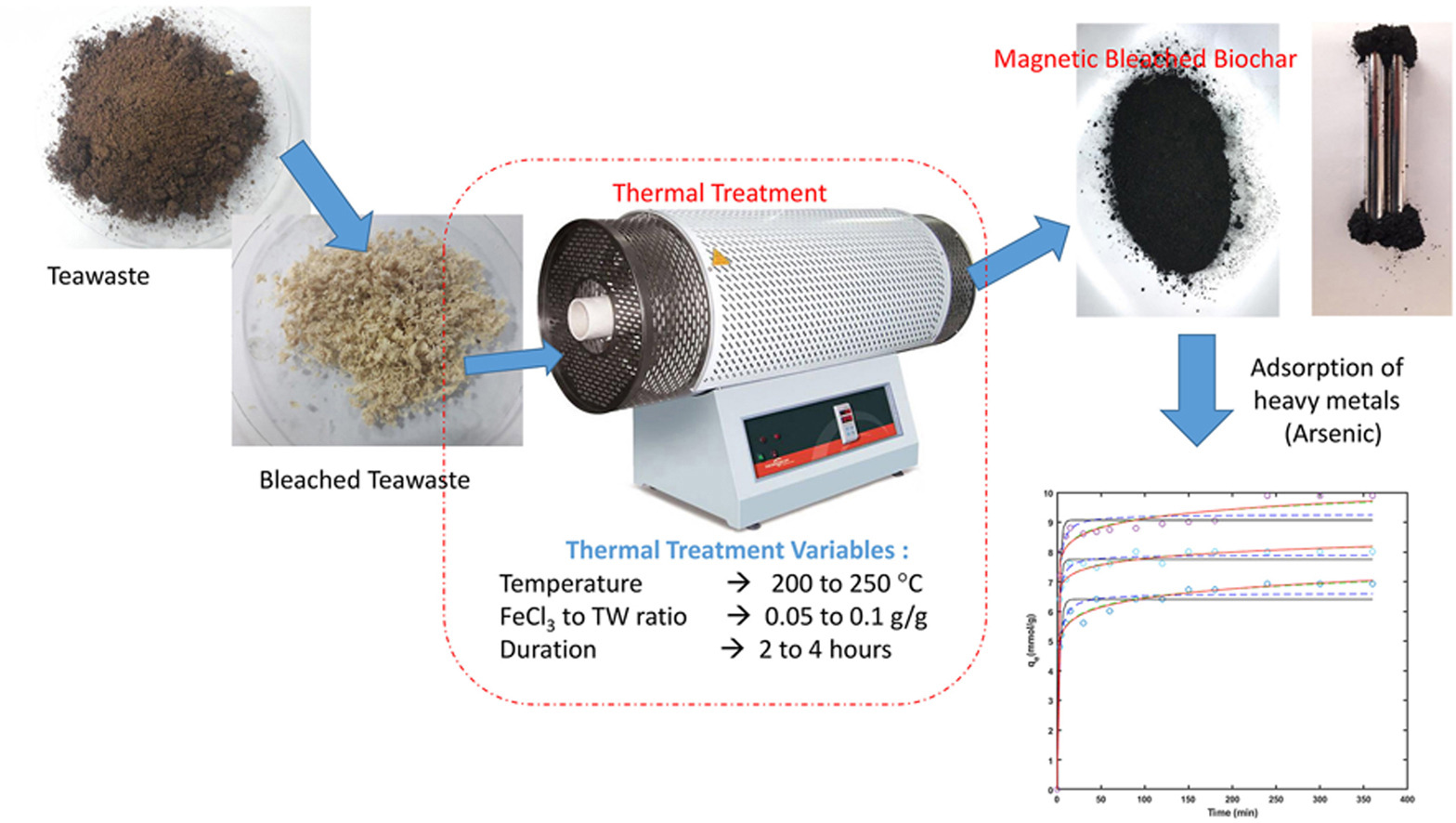
• Magnetic bleached biochar (MBBC) adsorbent material was successfully produced.
• Pseudo 2nd order kinetics model adequately describe As(III) removal and initial loading rate increases with temperature.
• The optimum dose for As (III) removal was 2.5 g/L.
• MBBC has a maximum Langmuir capacity 9.51 mmol/g (714 mg/g) at room temperature.
The primary objective of this research was to assess the potential of magnetic bleached biochar (MBBC) as a cost-effective adsorbent for arsenic removal. To achieve this, locally collected tea wastes underwent meticulous cleaning, bleaching, and modifications via thermal and chemical treatments. Both non-magnetic and magnetic biochar adsorbents were thoroughly characterized using Fourier transform-infrared spectroscopy (FT-IR) and thermo-gravimetric analysis (TGA). Subsequently, the adsorptive performance of MBBC in removing arsenic from wastewater samples was investigated, considering various crucial parameters such as adsorbent-adsorbate contact time, concentration of As, temperature, adsorbent dosage, and the regeneration-ability of the adsorbent. The experimental data for the adsorption process were best represented by the Langmuir isotherm, indicating its suitability for the MBBC adsorbent. Remarkably, the MBBC demonstrated a maximum Langmuir adsorption capacity of approximately 714 mg/g at room temperature, highlighting its efficiency as an arsenic adsorbent. Furthermore, the Lagergren's Pseudo-second order kinetic model proved to be the most suitable for describing the adsorption kinetics, confirming the chemisorption nature of the process. The results also indicated that the adsorption process is endothermic and feasible, suggesting its viability for practical applications. Taking all findings into account, the comprehensive analysis strongly supports the potential use of MBBC as a highly promising and cost-effective adsorbent for efficiently removing arsenic from aqueous samples. This research contributes valuable insights to the field of wastewater treatment and offers a sustainable and environmentally friendly solution for tackling arsenic contamination in water sources.