- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
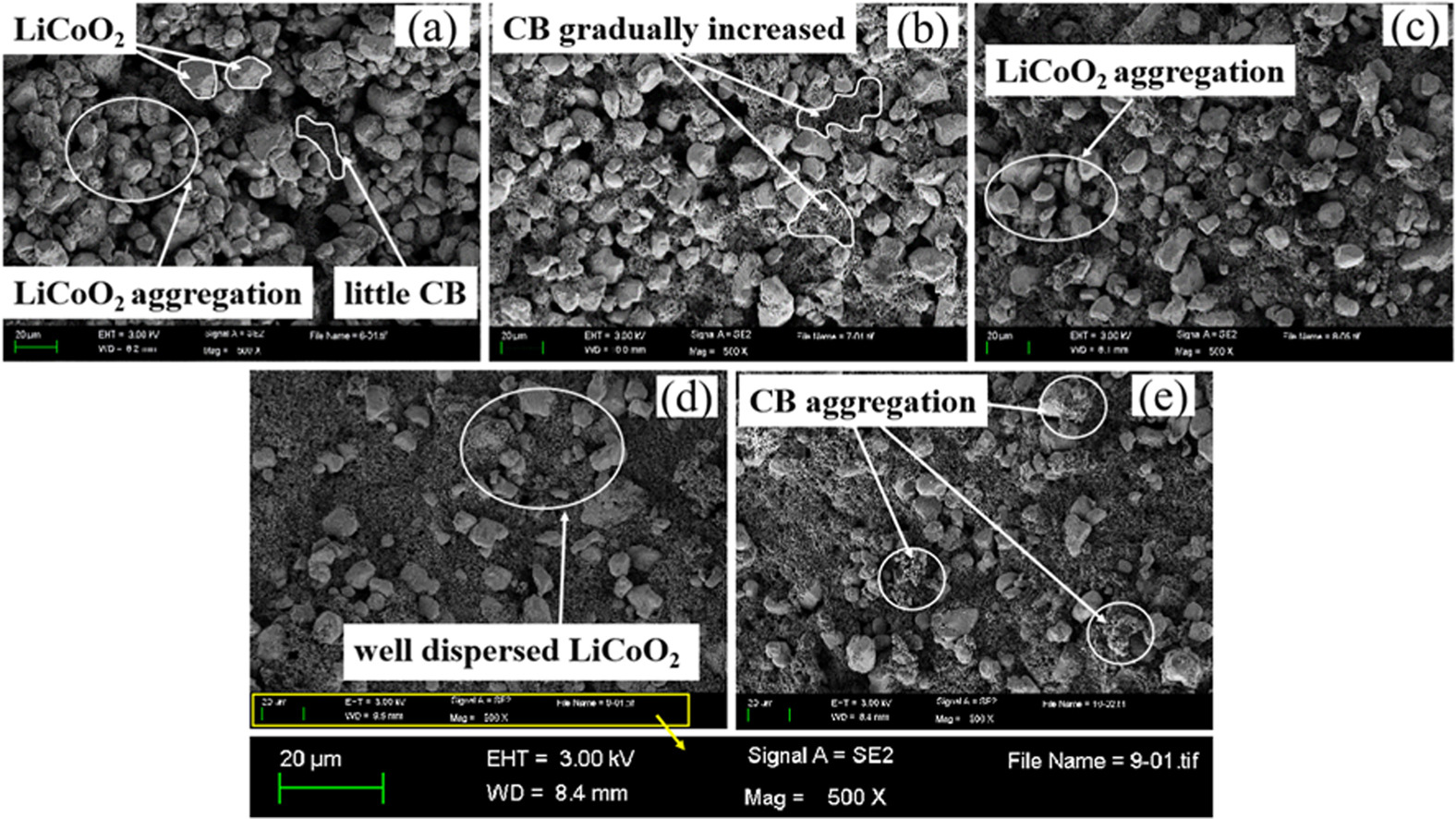
• LiCoO2 particles are completely coated by mixture of carbon black (CB) and poly vinylidene fluoride (PVDF) in the case of mPVDF:mCB = 5:10.
• A good conductive network structure is formed by coating thinner PVDF–CB double layer around LiCoO2 when t = 6 min.
• Cathode slurry prepared under conditions of both mPVDF:mCB = 5:10 and t = 6 min can improve performance of LIB.
This paper presents the effects of both poly vinylidene fluoride (PVDF)/carbon black (CB) ratio (mPVDF:mCB) and mixing time t on the dispersion mechanism of the cathode slurry of lithium-ion battery (LIB). The dispersion mechanism is deduced from the electrochemical, morphological and rheological properties of the cathode slurry by using electrical impedance spectroscopy (EIS), scanning electron microscopy and rheology methods, respectively. From the perspective of EIS method, static simulation models are established in the COMSOL Multiphysics software; meanwhile, the simulated results are used to verify the correctness of the electrochemical properties of the cathode slurry. As a result, the following conclusions are able to be obtained. Firstly, in the case of the mass ratio mPVDF:mCB = 5:10, LiCoO2 particles are completely coated by the mixture of CB and PVDF to form a stable polymer gel structure. Higher or lower mPVDF:mCB leads to the larger impedance and worse dispersion status for the cathode slurry. Secondly, when t = 6 min, a good gel-like conductive network structure is formed by coating the thinner evenly dispersed CB–PVDF double layer around LiCoO2 particles. Finally, a strategy regarding to both mPVDF:mCB and t in experimental scale is proposed, which has the capability of improving the performance of LIB.