- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Sulfur speciation in the conventional electrolyte composition is discussed.
• Thermodynamic and transport properties are used to rationalize electrolyte formulation.
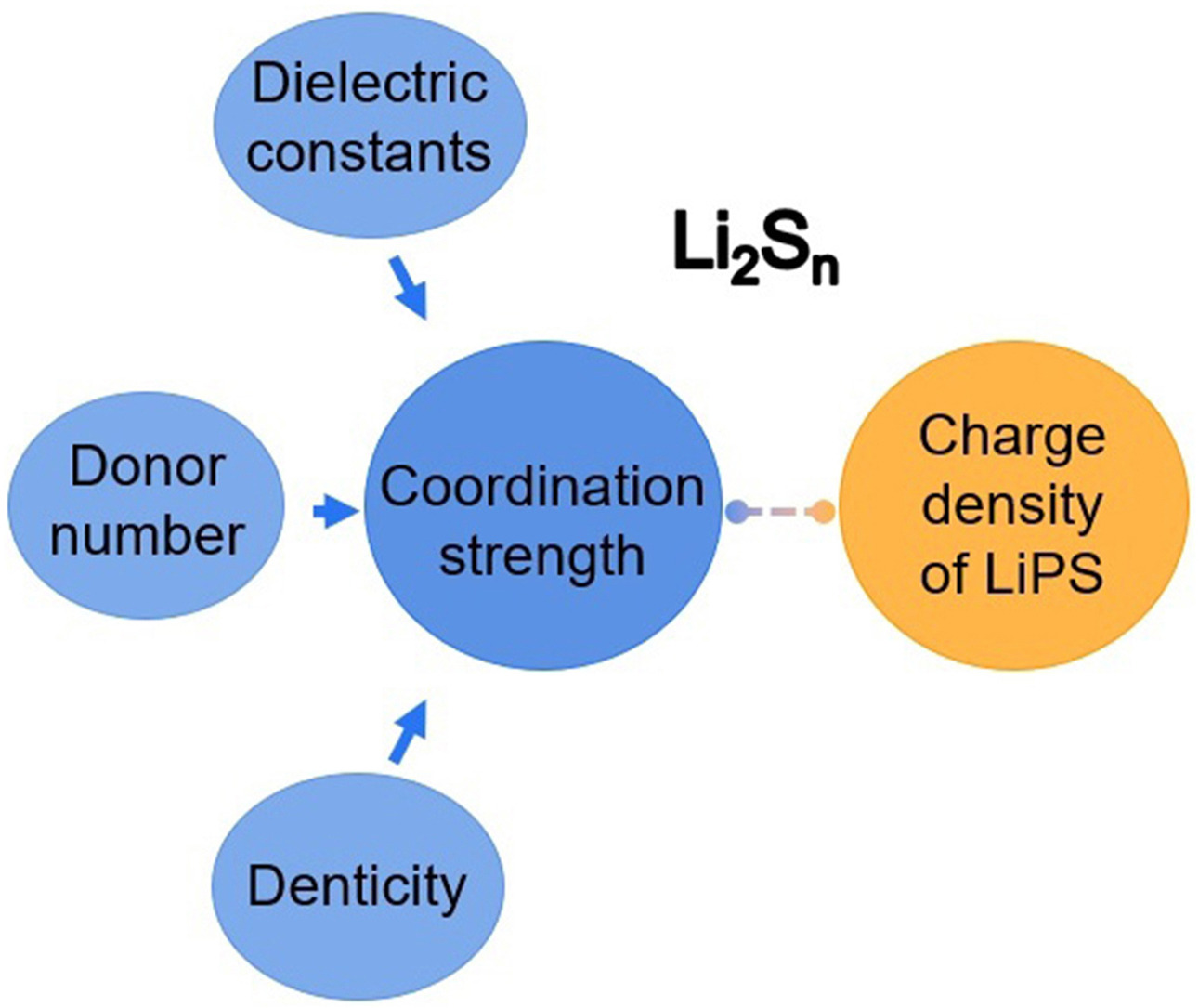
• The term coordination strength is employed to describe LiPS solvation.
• The chelating effect is identified as a critical descriptor to define LiPS structure.
Fingerprinting sulfur speciation in aprotic electrolytes is a key to understand fundamental chemistry and design well-performing lithium–sulfur (Li–S) batteries. Lithium polysulfide (LiPS) dissolution and deposition in ether-based electrolytes during redox reactions have been probed and established by spectroscopy and microscopy. However, detailed LiPS structure and solvation properties influenced by conventional and newly developed electrolytes remain elusive, which exert fundamental challenges and practical difficulties in decoupling battery performance from electrolyte volume. This perspective aims to provide timely information to uncover underlying mechanisms that rein in sulfur speciation by considering the charge density of LiPSs and the coordination strength of solvents/salts. The discussion starts with unlocking the baseline electrolyte formulation to investigate its role in LiPS formation and compatibility. After that, the term coordination strength is used instead of donor number and dielectric constant to describe interactions between solvents and LiPSs and to reveal LiPS structure evolution. This work is expected to encourage the discovery of new electrolyte working mechanisms to develop energy-dense and power-intensive Li–S batteries.