- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
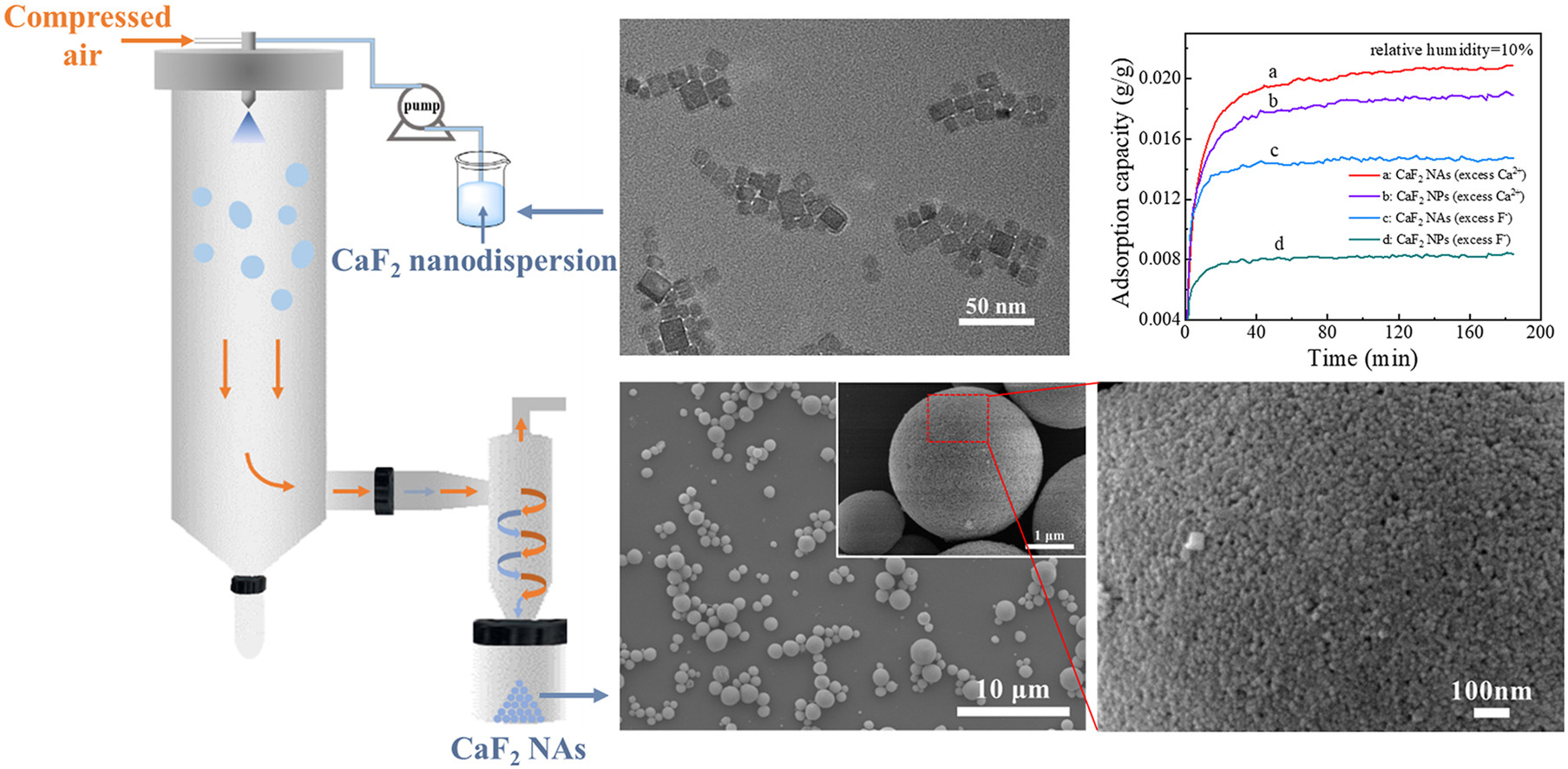
• CaF2 nanoaggregates (NAs) were efficiently constructed by spray drying technology.
• CaF2 nanoparticles (NPs) with two zeta potentials as building blocks were prepared.
• CaF2 NAs exhibited enhanced water vapor adsorption properties compared to CaF2 NPs.
Calcium fluoride (CaF2) is an ideal adsorbent for the dehydration of gaseous hydrogen fluoride (HF) containing water vapor. In this work, a novel CaF2 absorbent, spherical CaF2 nanoaggregates (NAs) with a closely packed structure, was proposed and efficiently fabricated by spray drying technology. As the building blocks of CaF2 NAs, the CaF2 nanoparticles (NPs) were prepared by the addition of excess calcium ions (Ca2+) or fluorine ions (F−) in the synthesis. The results indicated that the CaF2 NPs synthesized by excess Ca2+ and the corresponding NAs exhibited much better water vapor adsorption properties than their counterparts by excess F−, owing to higher zeta potentials. More importantly, whether excess Ca2+ or F−, CaF2 NAs had further enhanced water vapor adsorption capacity compared to primary CaF2 NPs, possibly owing to their unique nano-micro secondary structures and higher surface areas. This work has great potential in the development of high-performance absorbents for separating moisture from corrosive gas HF.