- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
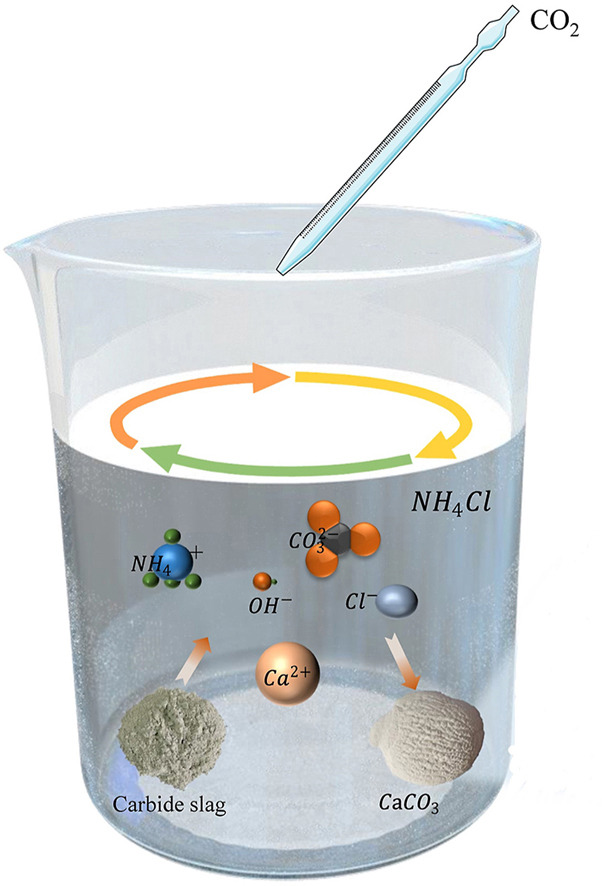
• High purity CaCO3 with different grain sizes, structures, and morphologies is successfully produced.
• NH4Cl is used to leach Ca2+ from carbide slag and can be recycled.
• The crystal phase, grain size, and morphology of CaCO3 could be controlled by adjusting the reaction conditions.
Based on the composition characteristics of carbide slag and the application of polyvinyl chloride, a method of preparing calcium carbonate with microstructure and nanostructure by using carbide slag as a raw material and ammonium chloride as a leaching agent was proposed. The factors for the preparation of calcium carbonate and the effects of different conditions on the crystal phase, grain size, and morphology of calcium carbonate were systematically studied. The results showed that the nanosized calcium carbonate was prepared at 60 mL/min, 25 °C, no additional ammonia, and 60 min. The product of spherical vaterite was in accordance with the relevant standards for the industrial precipitation of calcium carbonate. Moreover, the reuse of carbonation filtrate was realized. The crystal phase, grain size, and morphology of the carbonation product could be controlled by adjusting the reaction conditions. The manuscript provided a new idea for resource utilization of carbide slag and preparing nanocalcium carbonate.