- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
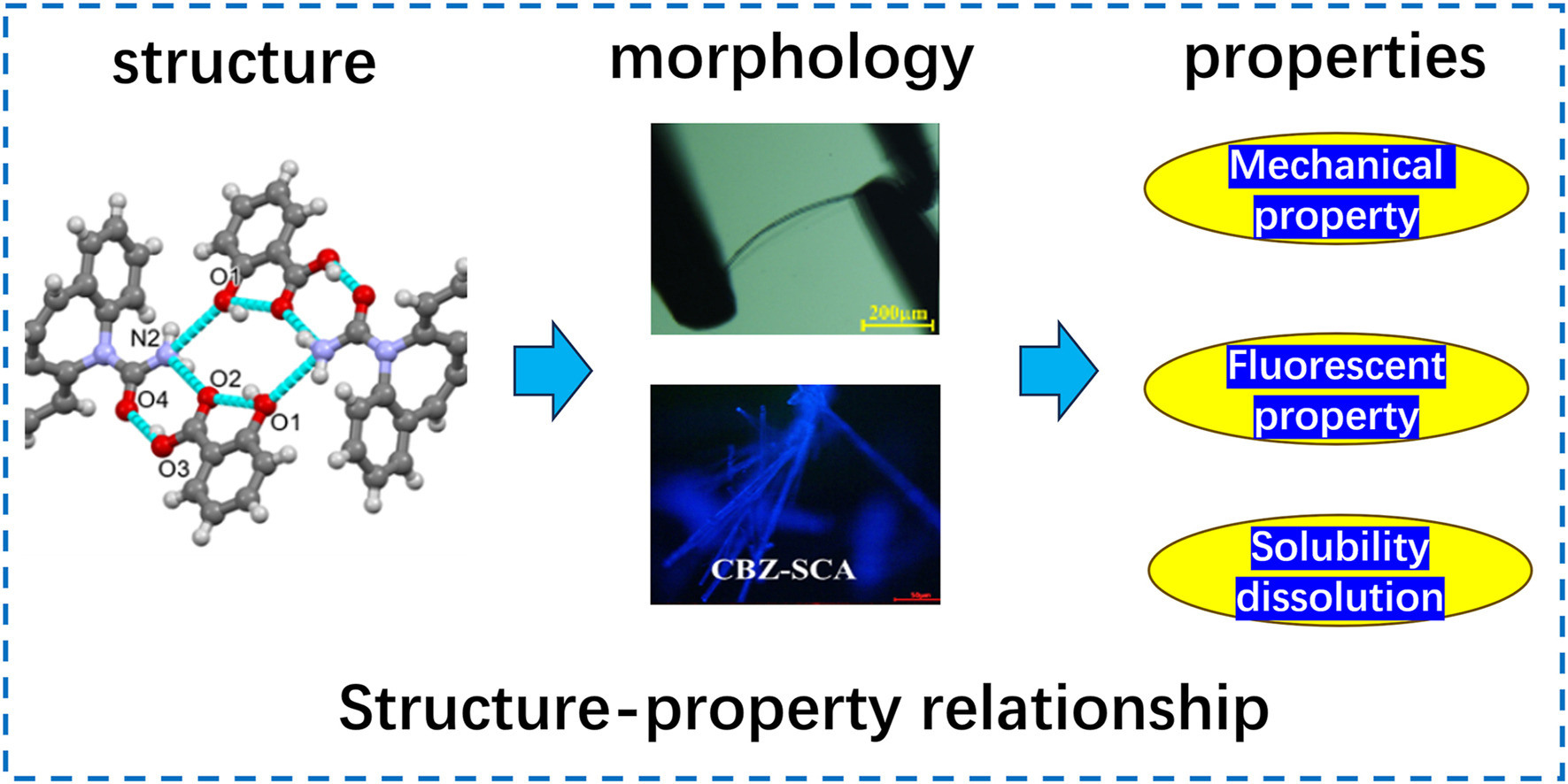
• Six cocrystals of carbamazepine were synthesized, and their structures were determined.
• Carbamazepine cocrystals possess better fluorescence performance than themselves.
• The dissolution rate of carbamazepine was enhanced by forming cocrystals.
• The mechanical property of carbamazepine was improved via cocrystal formation.
Carbamazepine (CBZ) is an anticonvulsant with very low water solubility, presenting as a white crystalline powder with poor mechanical properties and is hard to bend. To enhance CBZ's physicochemical properties, such as water solubility and mechanical properties, we selected six cocrystal coformers (CCFs): nicotinamide (NIC), benzamide (BZM), salicylic acid (SCA), fumaric acid (FMA), trimesic acid (TMA), and hesperetin (HPE). Six CBZ cocrystals were successfully prepared using the solution method. Fourier transform infrared spectroscopy (FT-IR), powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), and single crystal X-ray diffraction (SCXRD) were used to characterize the crystal structures and gain comprehensive insights into the six cocrystals. The mechanical, fluorescence, and solubility properties of the six cocrystals were tested. The results reveal that most of the prepared cocrystals exhibit improved water solubility and mechanical properties when compared to CBZ. Among them, the dissolution rate of cocrystals excluded from CBZ-HPE has increased by an average of 3 or 4 times compared to CBZ, while CBZ-HPE exhibits superior mechanical properties. Moreover, all six cocrystals possess better fluorescence performance than CBZ. We thoroughly evaluated the mechanical properties of the cocrystals through both experimental and theoretical approaches. This work provides a new direction for studying drug cocrystals to improve the physicochemical properties of drugs.