- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
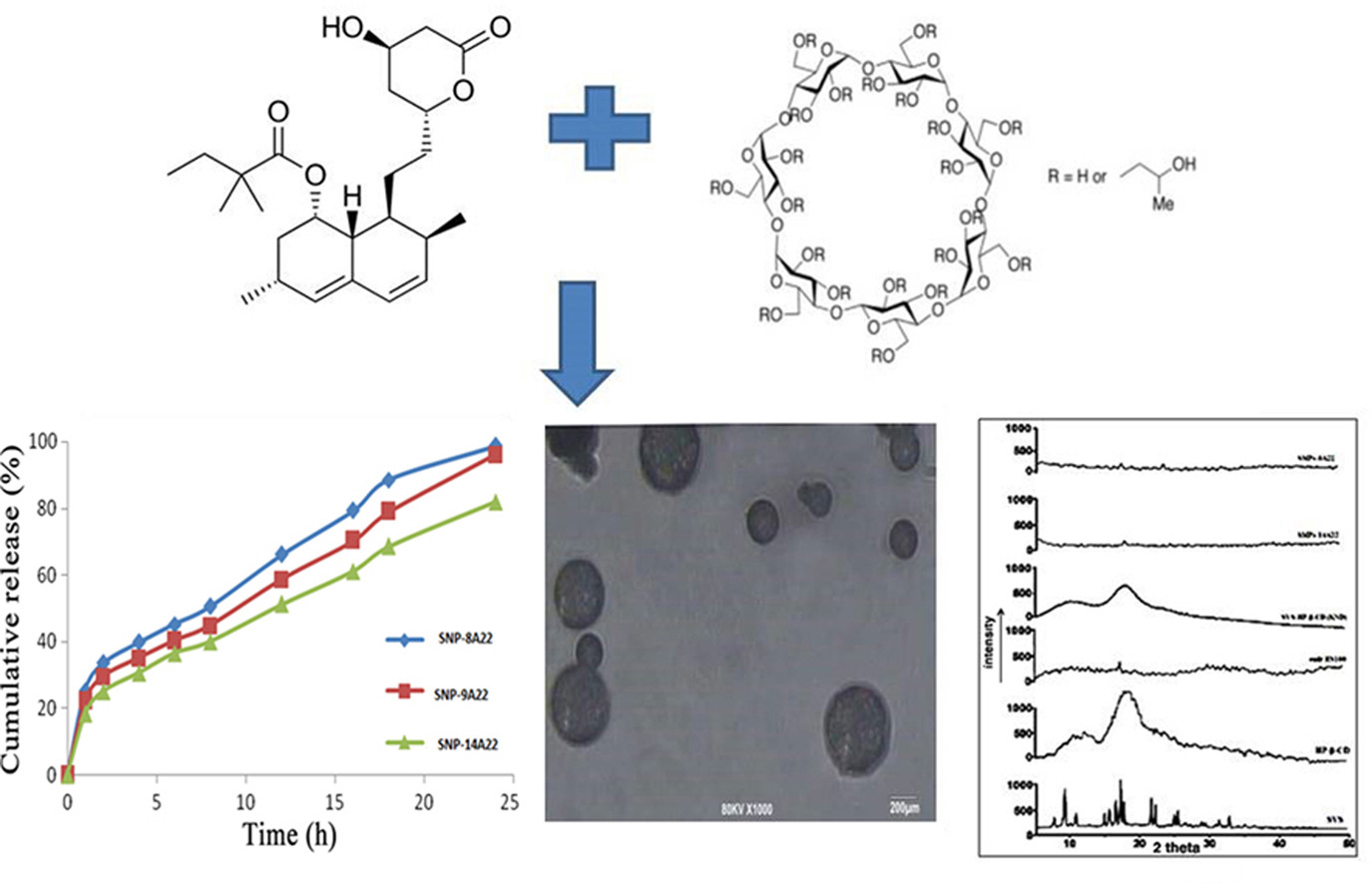
• Simvastatin is associated with poor solubility, first-pass metabolism, and short half-life.
• Optimization of formulation/process parameters was done through the use of variables.
• Nanoparticles were prepared by emulsification and a solvent evaporation technique.
• There was 2.93-fold decrease in total cholesterol.
• A 3.27-fold increase in triglyceride was during in-vivo study.
Simvastatin, a BCS class II drug, is associated with poor aqueous solubility, first-pass metabolism and short half-life. In the present work, nanoparticles were prepared and evaluated. Optimization of formulation and process parameters was done through the use of independent and dependent variables. Preliminary studies were done to determine suitable range of the concentration of Eudragit polymer (10%–30%) and the ratio of drug to polymer (1:1 to 1:5) for the formation of nanoparticles by emulsification and a solvent evaporation technique. Results revealed that the mean size of nanoparticles was affected by stirring speed from 5000 RPM, 8000 RPM, and 12000 RPM. The results of increase in association efficiency and percent yield with increase in amount of drug from 100 mg, 150 mg, and 200 mg in selected range were observed. In-vitro release studies showed that two formulations possess highest initial burst and slow sustained drug release. There was 2.93-fold decrease in total cholesterol and 3.27-fold increases in triglyceride level during in-vivo study.