- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Free porphyrins, phthalocyanines, covalent-organic, and metal complexes are used for water splitting (WS) electrocatalysts.
• Metal complexes in WS processes are widely represented by ruthenium coordination compounds.
• Artificial photosynthesis is based on copper-cobalt compounds, as phenantroline, porphyrazine, for commercial viability.
• Metal porphyrins/porphyrinates, loaded on inorganic materials are successfully applied in WS processes.
• Both HER and OER processes are available on porphyrin/phthalocyanine-based catalysts.
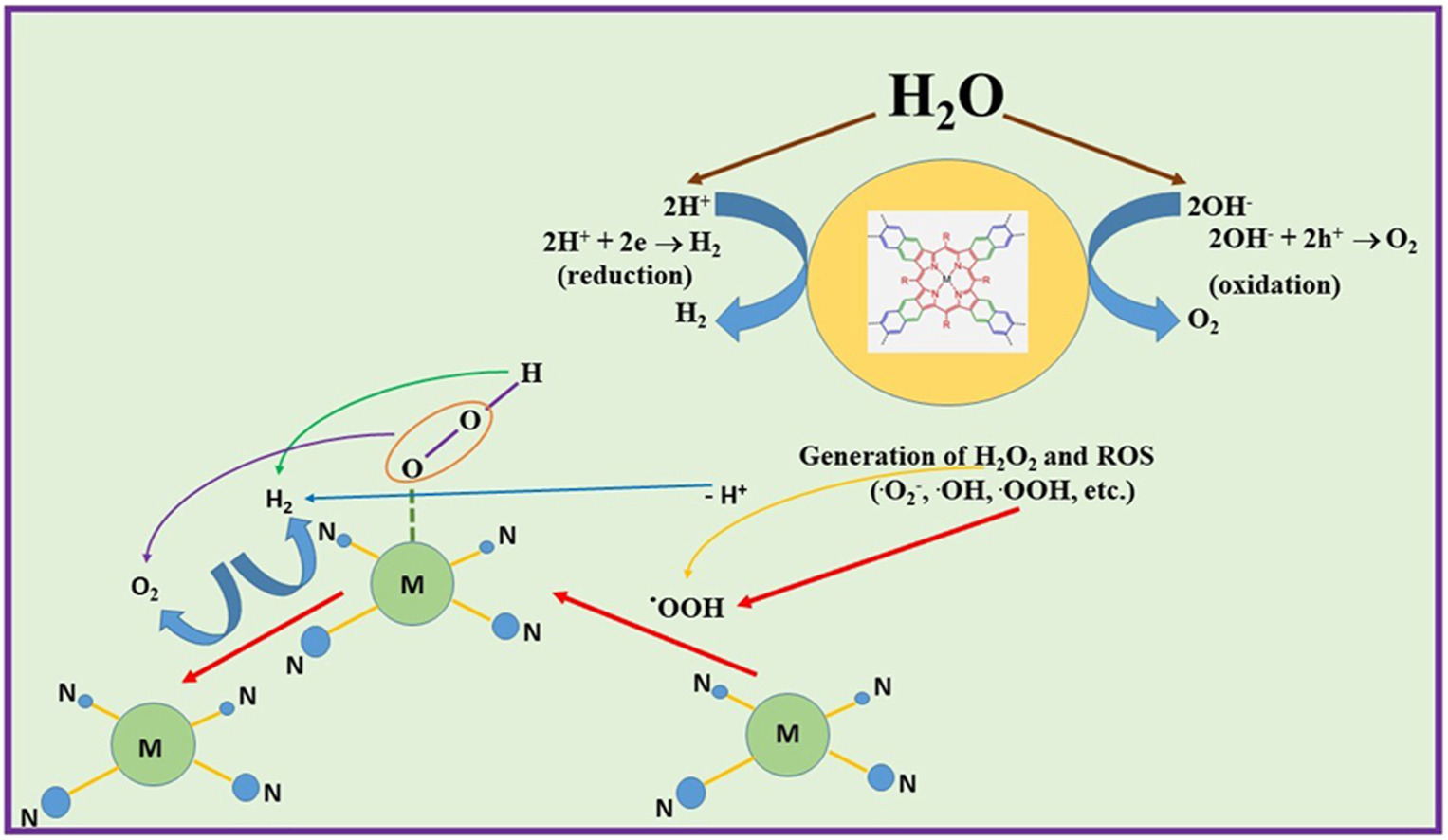
In this review, the possibilities of photochemical and electrochemical water splitting (WS) using porphyrins, phthalocyanines, related macrocycles, and their metal complexes are discussed. Significant efforts are being carried out in order to develop novel and cheap methods for water decomposition to generate inexhaustible greener energy sources (molecular hydrogen) instead of oil and gas. The processes of WS in heterogeneous and homogeneous media require novel photo- and electrocatalysts, in particular those on macrocycle basis. A high WS efficiency has been observed for porphyrins and phthalocyanines, showing their suitability both for HER and OER processes are available using these relative compounds for WS purposes.
Porphyrins can be used in photocatalytic and electrocatalytic WS processes both in their free forms or as metal porphyrins/porphyrinates, mainly loaded on various inorganic materials, such as Au NPs, C3N4, GaP, KTaO3, GaP, and so on, as part of porphyrin-containing polymers or on electrode surface as modifying coatings, being sometimes better than commercial catalysts, such as Pt/C. Photocatalytic H2 evolution can be considerably depended on the shape of porphyrin particles; in particular, porphyrin nanowires can yield up to 20 times more H2 than its powder. In certain cases, both HER and OER can be driven in the same electrolyte using metal porphyrins. The capacity of metal porphyrins to generate ROS (in particular, singlet oxygen 1O2) under ultrasonic treatment is known and can be successfully used for H2O2 and O2 generation. Phthalocyanines can be used in similar processes in free form or as composites with inorganic or organic counterparts, being immobilized on nanocarbons, metal oxides and salts, or simply dissolved in water acting in a homogeneous medium. An additional use of ultrasound can lead to better results due to the synergistic effect of the sonochemical treatment and light. Covalent-organic frameworks are also applied for WS purposes and are mainly based on a variety of N-containing ligands, such as triazine, β-ketoenamines, and β-ketoamines, bipyridine, as well as some S-containing ligands. Metal complexes in WS processes are widely represented by ruthenium coordination compounds, being suitable for future commercial artificial photosynthesis, as well by copper and cobalt coordination compounds with phenantroline, porphyrazine, and related derivatives, being used as electrocatalysts in the form of homogenous molecular films on glassy carbon electrode or as a part of a homogeneous photocatalytic water reduction system for HER processes. The analysis of the photo(electro)catalytic activity of these macrocycles indicated that the Covalent-organic frameworks are highly promising catalysts for H2 evolution from WS.