- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
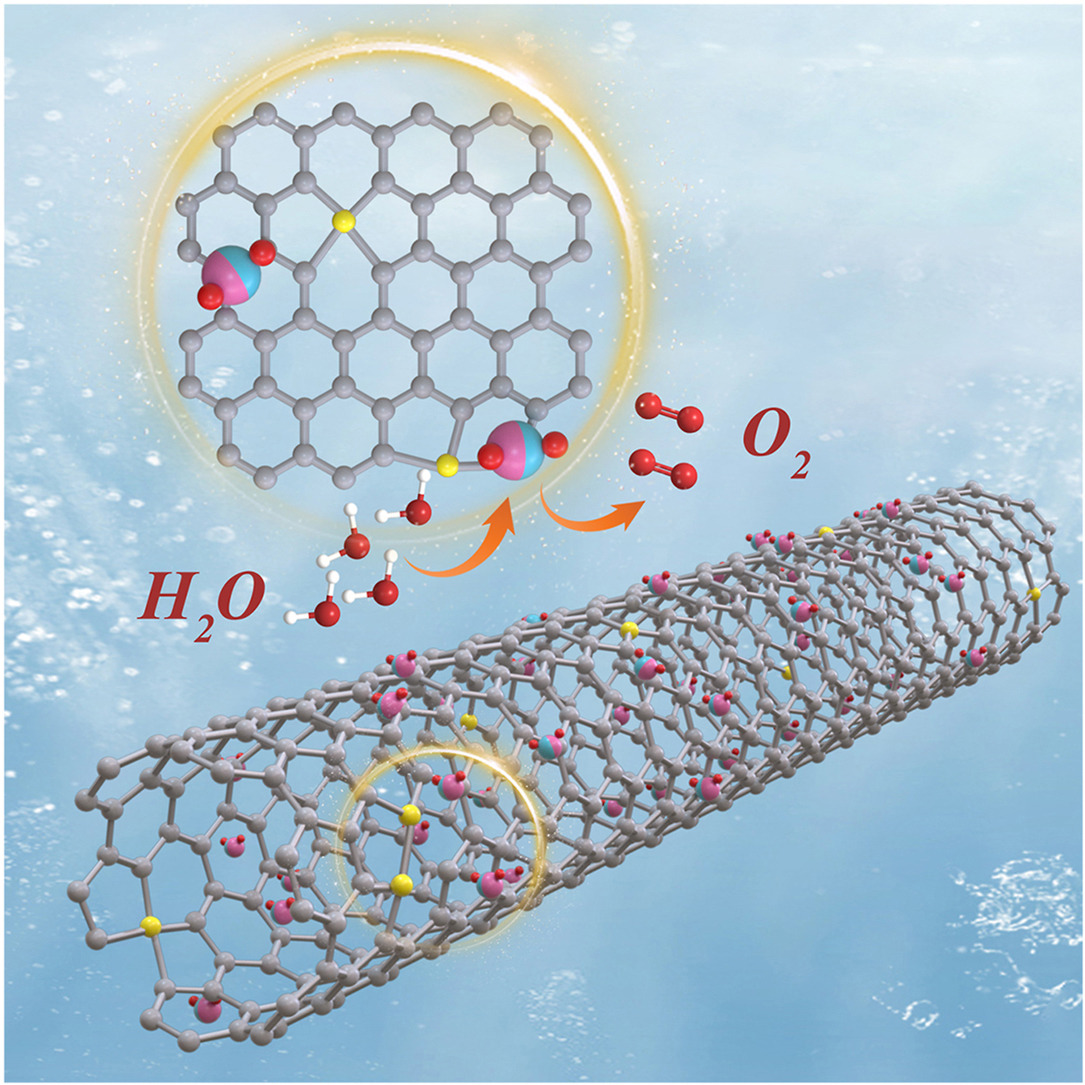
• Electron transport rate of the catalyst was enhanced by modulating the ordering degree of CNTs.
• Electronic structure of the active site Ru was jointly optimized by the introduction of the transition metals Co and CNTs.
• CoRuOx@CNTs-300 catalyst exhibited excellent acidic oxygen precipitation activity and stability.
Hydrogen production from proton exchange membrane water electrolysis is constrained by the sluggish kinetics of the anodic oxygen evolution reaction. RuO2 has attracted considerable attention due to its low reaction overpotential, but its inferior stability remains a major challenge. Herein, a strategy is proposed to enhance the catalytic activity and stability of CoRuOx nanoparticles by doping Co and regulating the ordering degree of carbon nanotubes (CNTs) by air annealing. It was found that the CoRuOx@CNTs-300 catalyst exhibited the best catalytic activity and stability when the annealing temperature was 300 °C. At the current density of 10 mA cm−2, the overpotential of this catalyst was only 201 mV, which was nearly 100 mV lower than that of commercial RuO2 (300 mV). Surprisingly, there was no significant increase in the overpotential when tested at a current density of 10 mA cm−2 for 50 h. The density functional theory calculations indicate that the high activity of the catalyst is due to the electronic coupling of CoRuOx nanoparticles and CNTs, and that the introduction of Co and CNTs improves the electronic structure and solvation energies of the Ru in the active site, dramatically increasing the structural stability.