- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
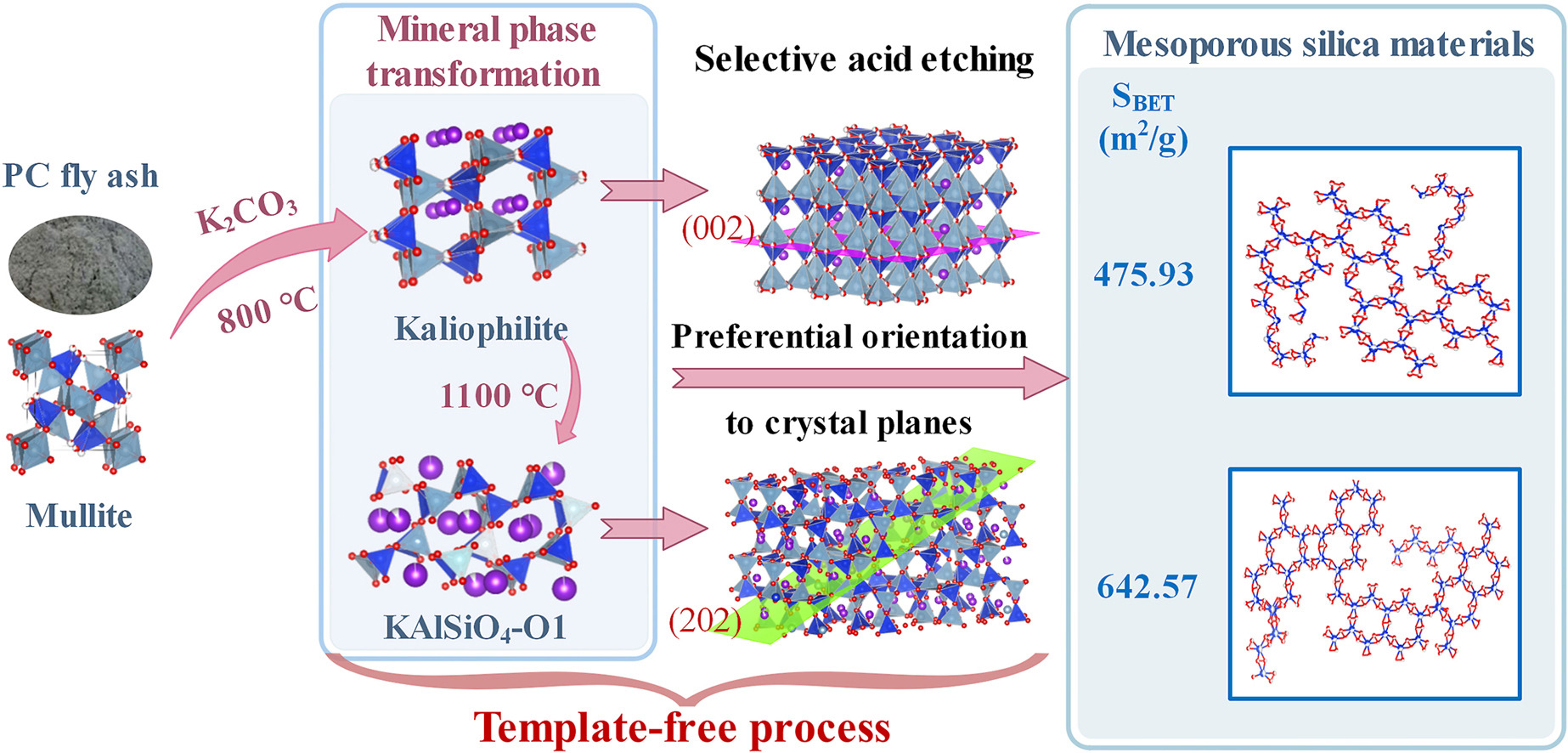
• Mesoporous silica materials were successfully produced from fly ash by the template-free process.
• Under the calcination with K2CO3, mullite gradually transforms into kaliophilite and KAlSiO4–O1 phase.
• With the increase of etching degree, the pore structure of the obtained silica-based materials is different.
• Mesoporous silica with specific surface area of 475.93 and 642.57 m2/g could be synthesized from activated fly ash.
• A cost-effective and large-scale process for mesoporous silica materials preparation from fly ash is provided.
Extraction of silica from fly ash to produce mesoporous silica materials is one of the most important utilization approaches. Mesoporous silica could not be synthesized on a large-scale by conventional sol-gel method. In this paper, facile preparation of mesoporous silica with controllable pore structure from fly ash by the template-free process via two steps of mineral phase transformation and selective acid etching was proposed. The influence of crystalline structure and acid etching degree on structure of as-synthesized mesoporous silica materials was revealed, as well as mechanism of crystalline structure transformation and pore structure formation. The results show that mullite and quartz could be transformed into acid-soluble kaliophilite when fly ash reacted with K2CO3 at temperature of 800–1100 °C. The hexagonal kaliophilite would be transformed into orthorhombic KAlSiO4–O1 phase when the temperature is controlled at 1100 °C. Mesoporous silica with specific surface area of 475.93 m2/g and 642.57 m2/g could be synthesized from activated fly ash with kaliophilite and KAlSiO4–O1 phase crystalline structure. By controlling the degree of acid etching, mesoporous silica materials with different pore structures can be obtained. This paper provides a cost-effective and large-scale process for the preparation of mesoporous silica materials with controllable pore structure from solid waste fly ash.