- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
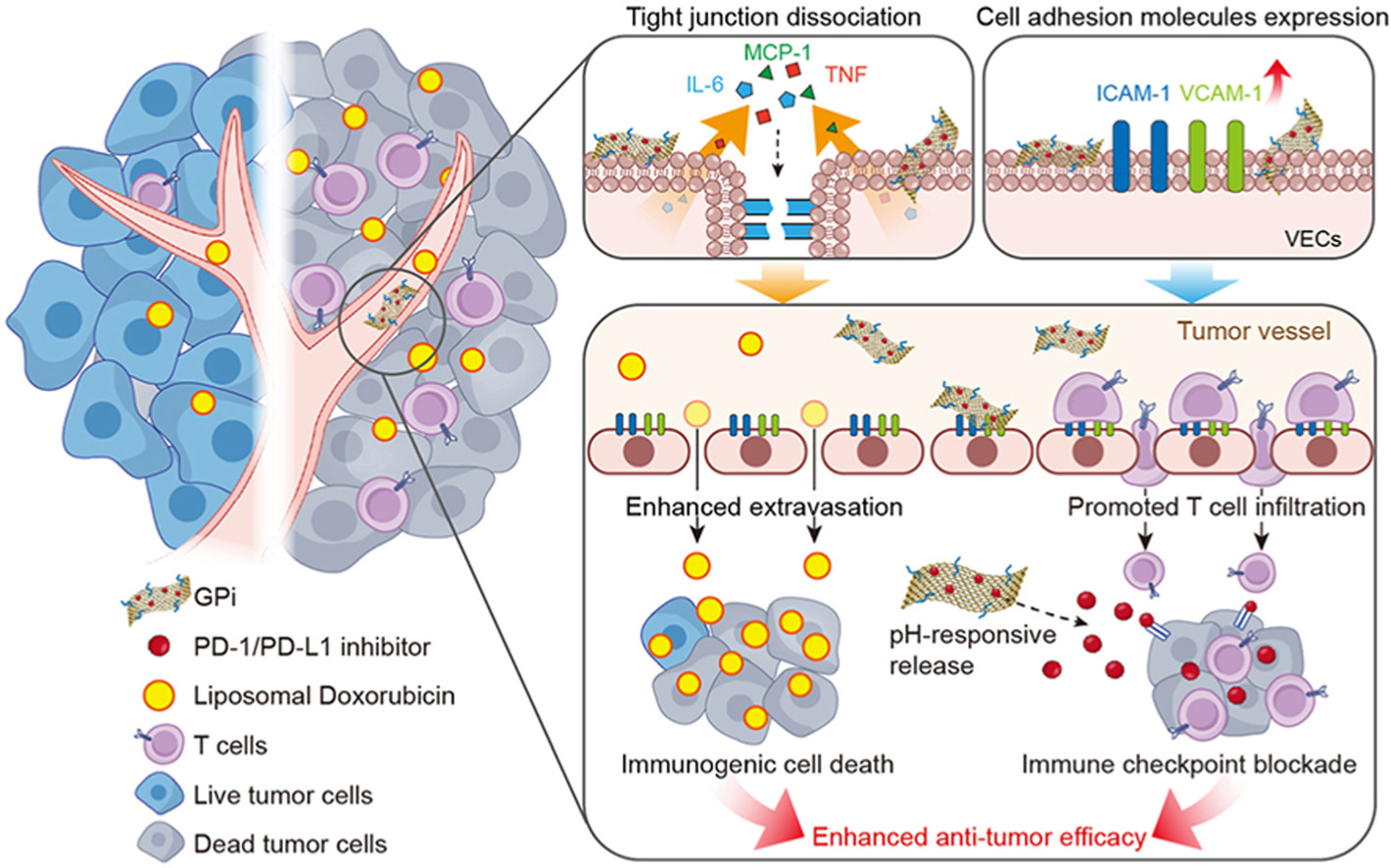
• We developed GPi to promote nanomedicine intratumoral accumulation and T cell infiltration by stimulating VECs.
• BMS-202 could be responsively released from GPi in tumor site to blockade PD-1/PD-L1 axis.
• The nanomedicine we used was clinical drug, thus conferring the favorable practical translational potential.
Immune checkpoint blockade (ICB) has emerged as a promising immunotherapeutic modality against cancer in the clinic. However, only 10–30% of patients respond to ICB, primarily due to poor immunogenicity and insufficient T cell infiltration in solid tumors. Herein, we presented an approach for high-performance cancer treatment using the programmed cell death protein-1 and programmed cell death ligand-1 (PD-1/PD-L1) inhibitor (BMS-202)-loaded PEGylated graphene oxide (GPi). On the one hand, GPi dissociated tight junctions of vascular endothelial cells (VECs) in tumor, thus promoting the extravasation and intratumoral accumulation of liposomal doxorubicin (LipDox), which then effectively induced immunogenic cell death of tumor cells. On the other hand, GPi also stimulated VECs to upregulate the expression of cell-cell interaction molecules, such as intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1, which facilitated the infiltration of T cells in tumor. Beyond acting as a stimulator of VECs, GPi could exert responsive release of BMS-202 under the acidic tumor microenvironment and blockade PD-1/PD-L1 axis in tumors. Finally, the alternating administration of GPi and LipDox effectively inhibited tumor growth in a 4T1 tumor model, providing a novel treatment mode for chemo-immunotherapy.