- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
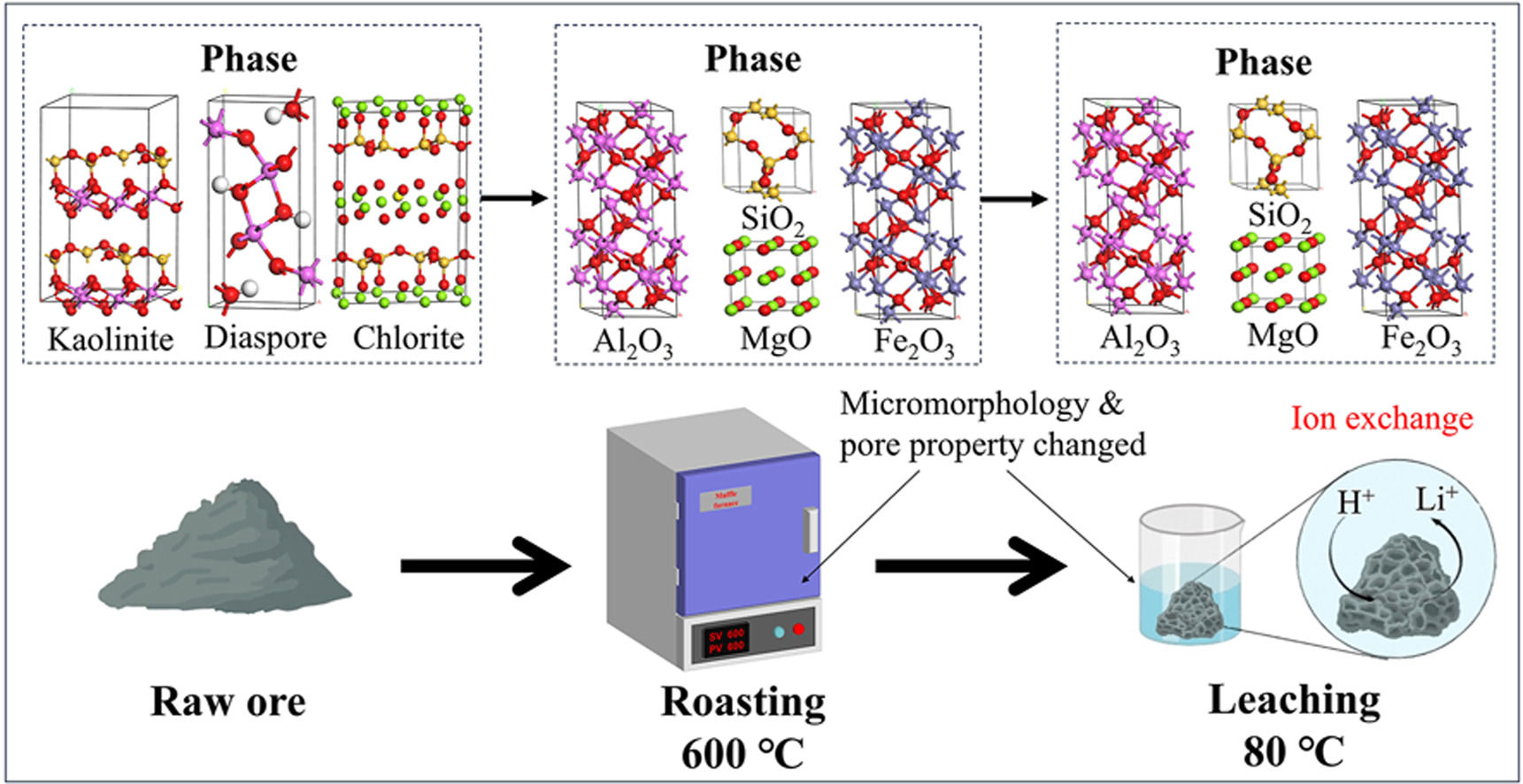
• Leaching rate of lithium was 97.83% by mixed acid.
• Roasting and leaching temperature was 600 and 80 °C respectively.
• Chemical reaction occurred during roasting and leaching.
• Phase changed during roasting.
• Micromorphology & pore properties changed during roasting and leaching.
A roasting-leaching test was carried out for the efficient utilization of clay-type lithium ore in the central region of Yunnan province. The test used the mixed acid of sulfuric acid and phosphoric acid as the leaching agent. Under the conditions of roasting temperature of 600 °C, roasting time of 1 h, liquid-solid ratio of 5:1, volume ratio of H2SO4 solution to H3PO4 solution of 45:5, leaching time of 2 h and leaching temperature of 80 °C, the leaching rate of lithium was as high as 97.83%. The leaching mechanism was studied by SEM, pore property analysis, XRD and XPS. It was found that the morphology of the ore changed obviously after roasting and leaching, and a certain degree of collapse and fragmentation occurred, which provided favorable spatial conditions for the leaching of lithium. The porosity, total intrusion volume and total pore area also increased after roasting and leaching, thus promoting the leaching of Li+. The results showed that chemical reaction taken placed during the roasting and leaching. The phase of the sample changed from chlorite, kaolinite and diaspore (boehmite) mainly to corundum, hematite, periclase and quartz after roasting. However, after leaching, no new phase was produced in the ore sample, and no S and P elements were found on the surface of the ore sample, indicating that the leaching mechanism of lithium might be the ion exchange between H+ and Li+.