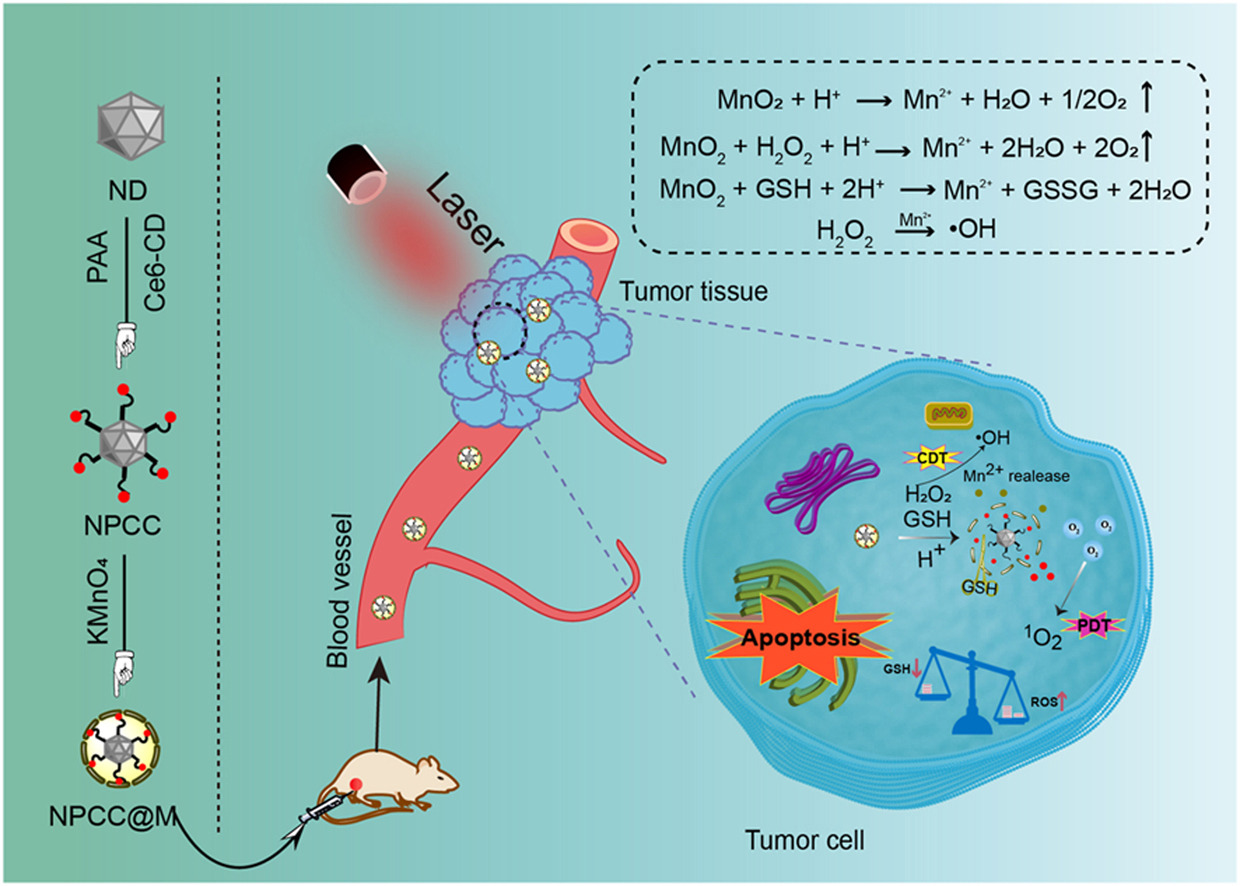
• The NPCC@M nanoparticles confer Chlorin e6 targeting selectivity and enhanced photodynamic therapy.
• External MnO2 of NPCC@M catalyzes the endogenous H2O2 inside tumors to produce O2, alleviating tumor hypoxia.
• GSH consumption through the MnO2 and the destruction of disulfide bonds breaks redox homeostasis inside tumors.
The generation of reactive oxygen species (ROS) at the tumor site to induce destruction is emerging as a novel strategy for cancer treatment, which involves photodynamic therapy (PDT). Nevertheless, tumors typically create a hypoxic environment and are equipped with an endogenous antioxidant defense system that could potentially impede the efficiency of the therapeutic approach. To overcome these drawbacks, herein, a tumor microenvironment-responsive the ND-PAA-CD-Ce6@MnO2 (NPCC@M) delivery system was fabricated by disulfide bond coupling chlorin e6 (Ce6) to nanodiamond (ND) and further wrapped by MnO2 nanosheets to facilitate PDT. The use of disulfide bond not only stabilizes Ce6 in the blood circulation to prevent premature leakage, but also destroys the antioxidant barrier of overexpressed glutathione (GSH) in tumor cells. Moreover, the outer MnO2 was rapidly degraded by the endogenous hydrogen peroxide (H2O2) in the acidic pH and GSH within the tumor cells, which leads to an abundance of O2 and while increases the level of 1O2 under laser irradiation. The results eventually broke the redox homeostasis and attenuate hypoxia, thereby inducing apoptosis and necrosis of tumor cells. Detailed in vitro and in vivo biological effect has revealed a good biosafety profile and a high tumor suppression effect. Such a novel ND-based system with tumor microenvironment-modulating capability to elevate oxygen content and promote GSH consumption in tumor cells opens new opportunities for enhanced ROS treatment paradigms.