• A new-style 3D flower-like MgAl-LDH@UiO-66-NH2 adsorbent was first constructed.
• MgAl-LDH@UiO-66-NH2 composite exhibits adsorption performance toward levofloxacin.
• MgAl-LDH and UiO-66-NH2 structure is beneficial to expose more active sites and facilitate levofloxacin capture.
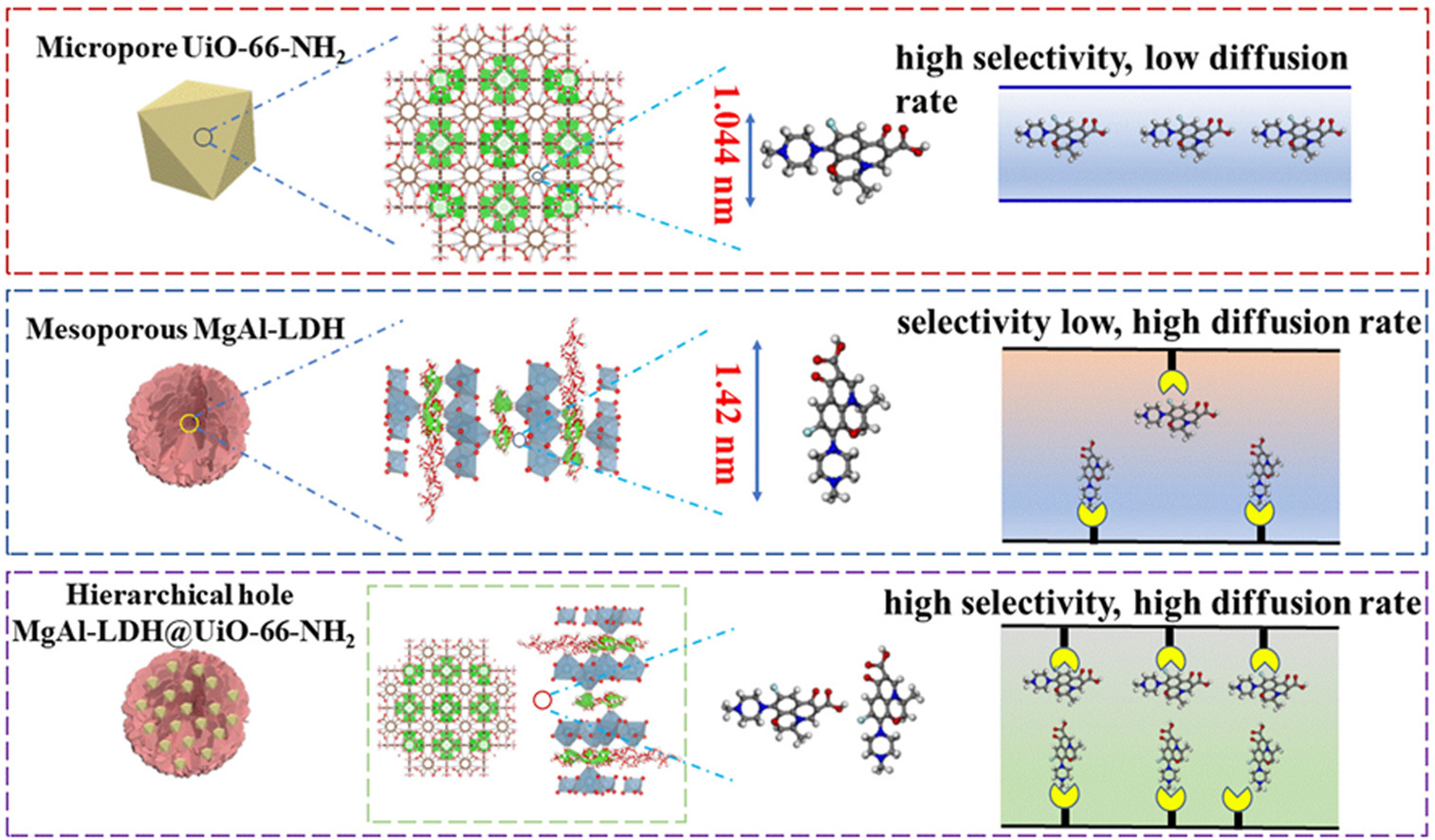
The adsorption performances of individual adsorbent such as UiO-66-NH2 and MgAl-layered double hydroxides (MgAl-LDH) toward levofloxacin (LVX) is less satisfactory due to the disappointing uptake capacity and adsorption efficiency. Herein, a 3D flower-like composite adsorbent prepared via iterative hydrothermal synthesis method anchoring UiO-66-NH2 nanoparticles onto the MgAl-LDH surface. Attributed to the synergistic effects of UiO-66-NH2, and MgAl-LDH, the MgAl-LDH@UiO-66-NH2 exhibits an outstanding adsorption performance toward LVX. Typically, the maximum LVX uptake capacity over MgAl-LDH@UiO-66-NH2 reached 268 mg/g, which was higher than that of the individual adsorption materials UiO-66-NH2 (41 mg/g), MgAl-LDH (140 mg/g), and some adsorbents reported in previous literature. The rapid adsorption process can reach equilibrium within 20 min, which conformed well to the pseudo-second-order kinetics and the Langmuir isotherm model. Adsorption thermodynamic result also indicated that the adsorption was spontaneous and exothermic, simultaneous coexisting physical and chemical adsorption interactions. Importantly, the MgAl-LDH@UiO-66-NH2 still maintained excellent adsorption capacity with a slightly decrease after fifth cycle, exhibiting a remarkable reuse and stable performances. This work provides a highly novel strategy to prepare recyclable composite adsorbent for the LVX capture from aqueous solution.