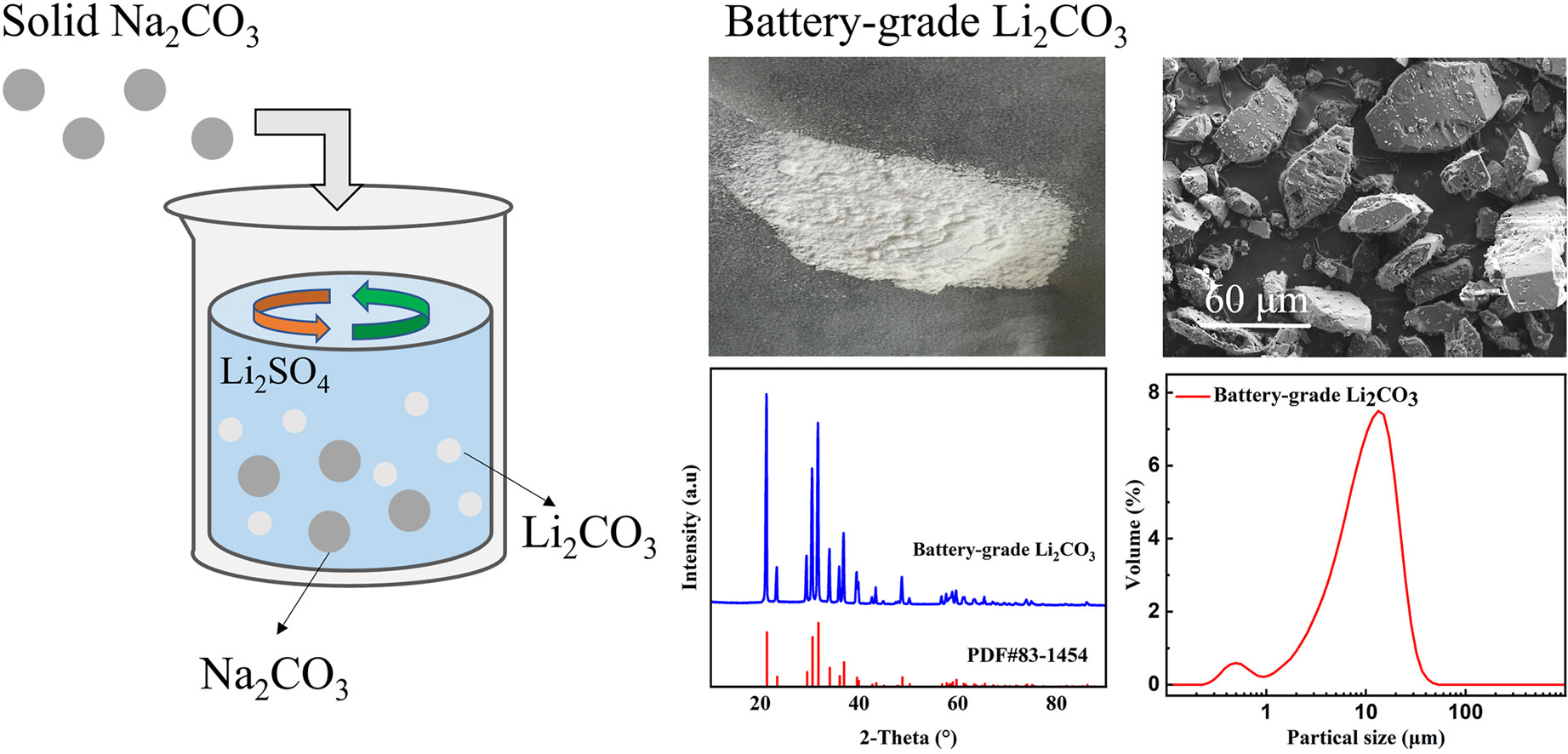
• Battery-grade Li2CO3 is successfully produced.
• Na2CO3 is added as solid and the single step recovery rate is increased to over 93%.
• Solid-liquid reaction crystallization mechanism of Li2CO3 is elucidated through apparent kinetic calculations.
• Effects of different reaction conditions on the recovery rate, purity, and morphology of Li2CO3 products are explored.
Lithium carbonate (Li2CO3) stands as a pivotal raw material within the lithium-ion battery industry. Hereby, we propose a solid-liquid reaction crystallization method, employing powdered sodium carbonate instead of its solution, which minimizes the water introduction and markedly elevates one-step lithium recovery rate. Through kinetic calculations, the Li2CO3 solid-liquid reaction crystallization process conforms by the Avrami equation rather than shrinking core model, which means the dissolution rate of Na2CO3 is the most important factor affecting the reaction process. The effects of reaction conditions such as temperature and stirring speed on the Li2CO3 precipitation behavior were evaluated. The results indicated that temperature is a most essential parameter than other reaction conditions or stirring speed. The exceptional 93% recovery of Li2CO3 at 90 °C with a remarkable purity of 99.5% was achieved by using 1.2 M ratio of Na2CO3/Li2SO4. This method provides a new idea for the efficient preparation of battery-grade Li2CO3.