• Layered TiO2 offers a high lithium-ion diffusion coefficient and high conductivity.
• In-situ characterization techniques elucidate the electrochemical reaction mechanism.
• Layered TiO2 exhibits pseudocapacitive effects, resulting in superior electrochemical performance.
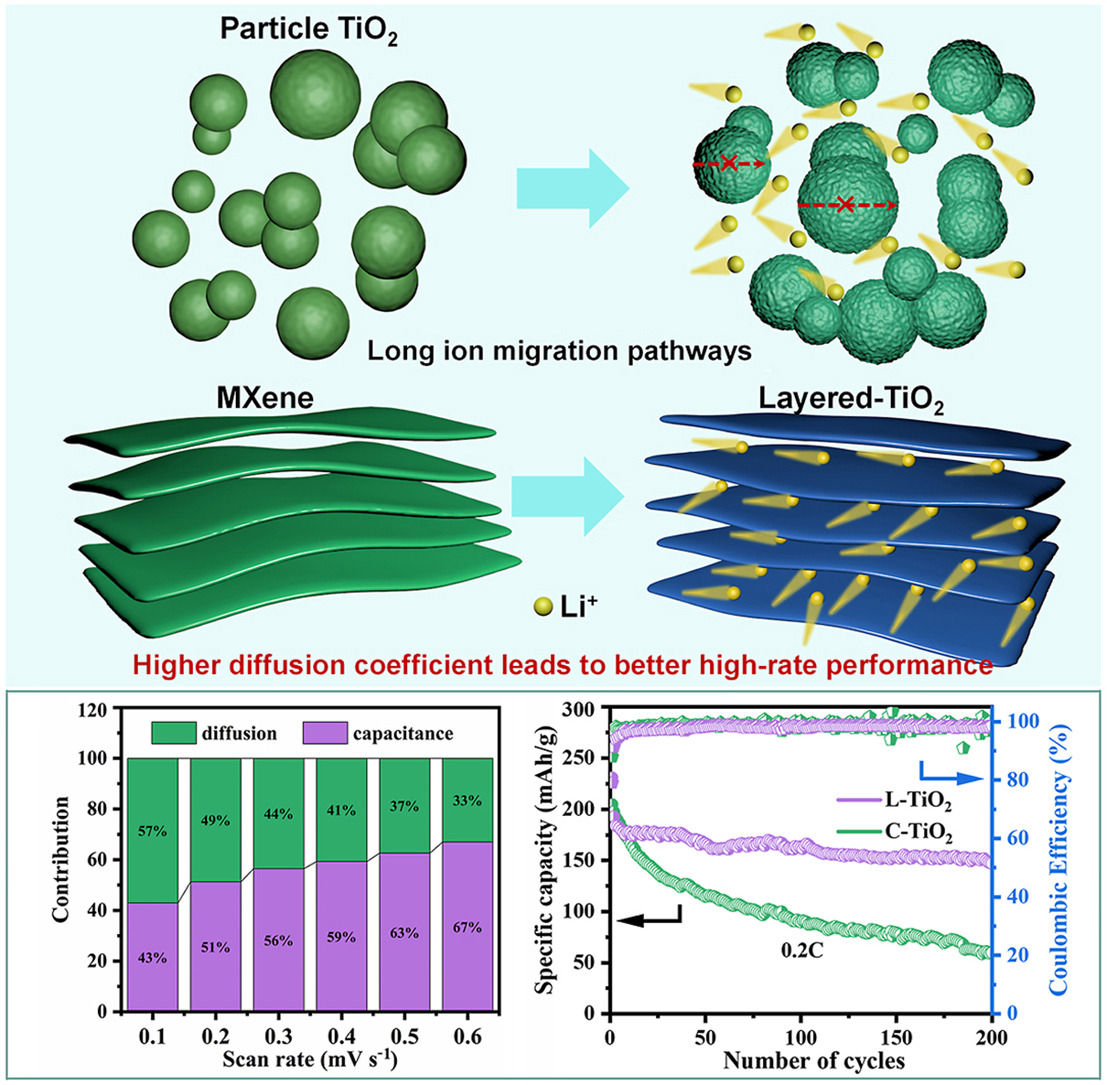
Due to the typical intercalation-deintercalation mechanism, TiO2 holds great promise as a sustainable anode for next-generation lithium-ion batteries (LIBs). However, commercial TiO2 (C–TiO2) is granular and shows slow ionic conductivity, which greatly hinders its development due to sluggish kinetics, leading to low reversible capacity and inferior rate capability. In this study, a two-dimensional layered TiO2 (L-TiO2) anode is prepared via a one-step calcination process, which can effectively shorten the lithium ions diffusion path and improve its lithium ions conductivity. We elucidated the enhanced electrochemical performance of L-TiO2 as an anode in LIBs through pseudocapacitive acceleration of lithium ions intercalation and deintercalation using various characterization techniques, including different scan rate cyclic voltammetry tests, in situ electrochemical impedance spectroscopy, in situ Raman spectroscopy, and in situ X-ray diffraction. In comparison to C–TiO2 material, L-TiO2 material showcases remarkable electrochemical performance, achieving a capacity of 166 mAh/g after 100 cycles at 0.1 C. Additionally, the lithium-ion diffusion coefficient calculated for the L-TiO2 is two orders of magnitude greater, underscoring its potential as a negative electrode material for LIBs.