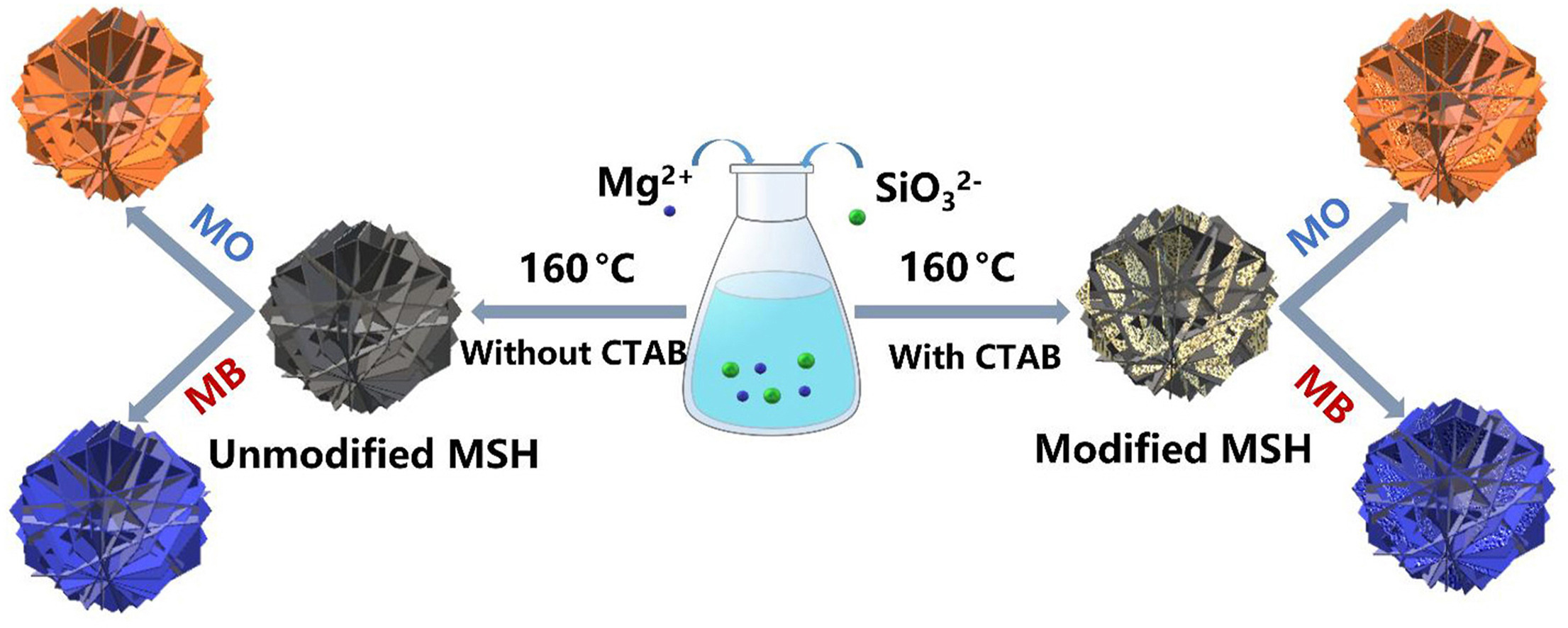
• Hierarchical flower-like magnesium silicate hydrate microspheres were fabricated.
• Formation mechanism and adsorption behavior of microspheres has been investigated.
• Methyl orange removal follows CTAB, and methylene blue adsorption is related to Si.
Hierarchical porous magnesium silicate hydrate (MSH) microspheres composed of sheets are successfully developed under facile conditions using a hard template. The role of hexadecyltrimethylammonium bromide (CTAB) on the formation and adsorption behavior was also observed for the methyl orange and methylene blue. The formed MSH possesses a surface area of 453.24 m2/g, an average pore size of 6.38 nm, and a pore volume of 0.75 cm3/g without CTAB. Based on the role of CTAB and the change in the ratio of Mg/Si, the MSH retained its sphere-like structure with a variation in pore parameters. The formed MSH was used as an adsorbent to remove methylene blue and methyl orange. The pseudo-second-order kinetic and Langmuir Isotherm models are well-fitted, with a 256.4 mg/g removal capacity and 84.2 mg/g for methylene blue and methyl orange, respectively. The modified MSH with CTAB played a positive role for the methyl orange and a negative role for the methylene blue regarding removal performance.