• NiCoFeCuS high-entropy electrocatalyst without any noble metals was prepared and applied in alkaline OER.
• Catalytic active sites are produced by electron density redistribution in high-entropy and sulfurization.
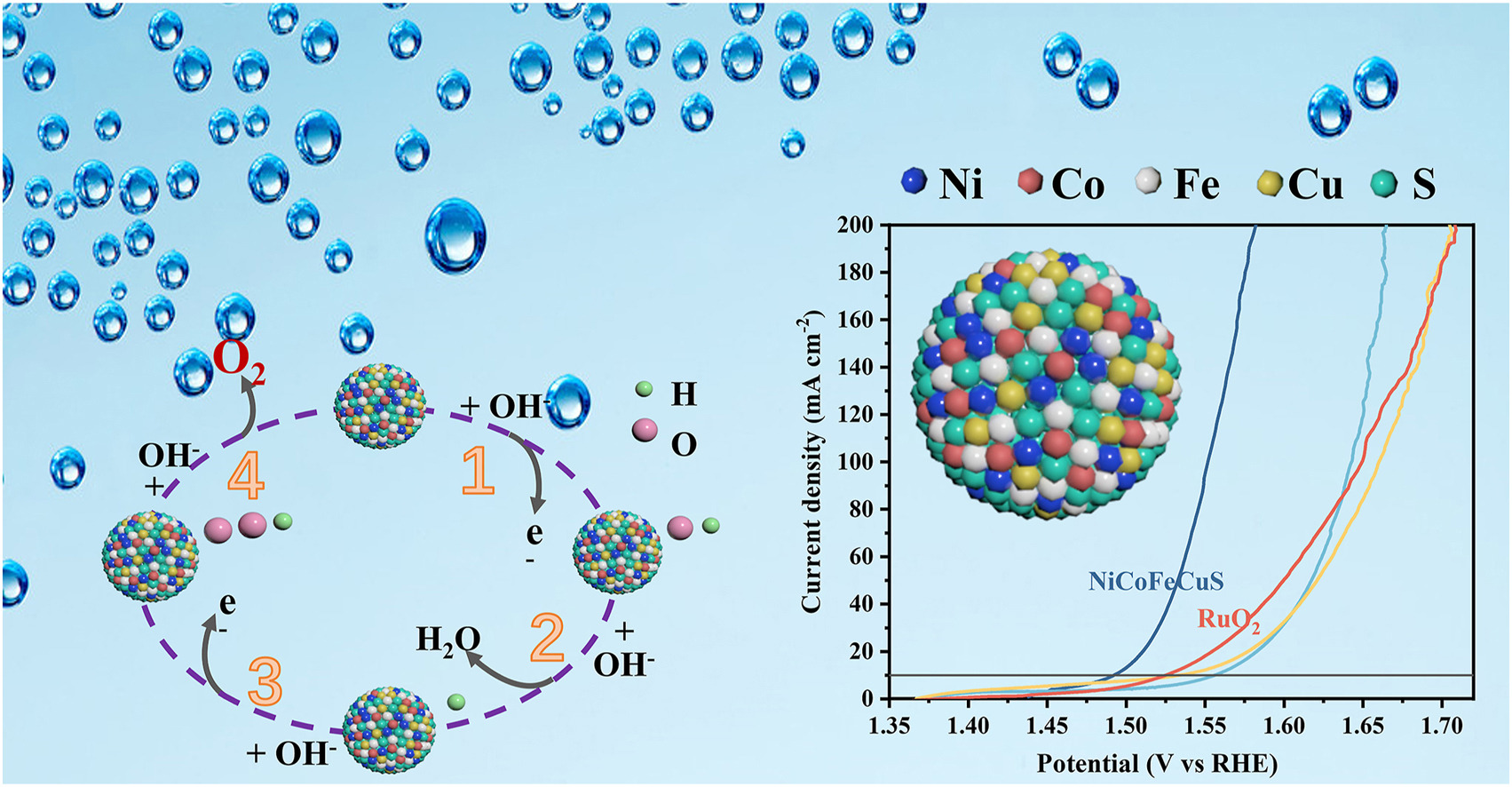
• NiCoFeCuS exhibits 261 mV overpotential and 57.97 mV dec−1 Tafel slope @ 10 mA cm−2, 88% of commercial RuO2 only.
Highly active and cost-effective oxygen evolution reaction electrocatalysts have become essential to replace commercial electrocatalysts that rely on rare noble metals. High-entropy sulfide nanomaterials, characterized by abundant randomly distributed elements and inherent stability, possess significant potential for applications. However, challenges such as uneven composition, partial oxidation, or imprecise synthesis control still remain in the materials preparation. Herein, a simple and effective two-step hydrothermal method was employed to synthesize NiCoFeCuS nanoparticles supported on foam nickel substrate. With the catalytic active sites produced by electron density redistribution in high-entropy and sulfurization, NiCoFeCuS exhibits excellent alkaline OER performance, with an overpotential of 261 mV and a Tafel slope of 57.97 mV dec−1 at the current density of 10 mA cm−2, which is only 88% of commercial RuO2 without any noble metals.