• First-principles found the most stable Fe/Mn ratio for P2-type Fe–Mn cathode materials in sodium-ion batteries.
• Simulating structural changes during sodium extraction, partial density of states and Bader charges were computed.
• This reveals Fe–Mn synergy in material, guiding rational design of sodium-ion battery cathodes.
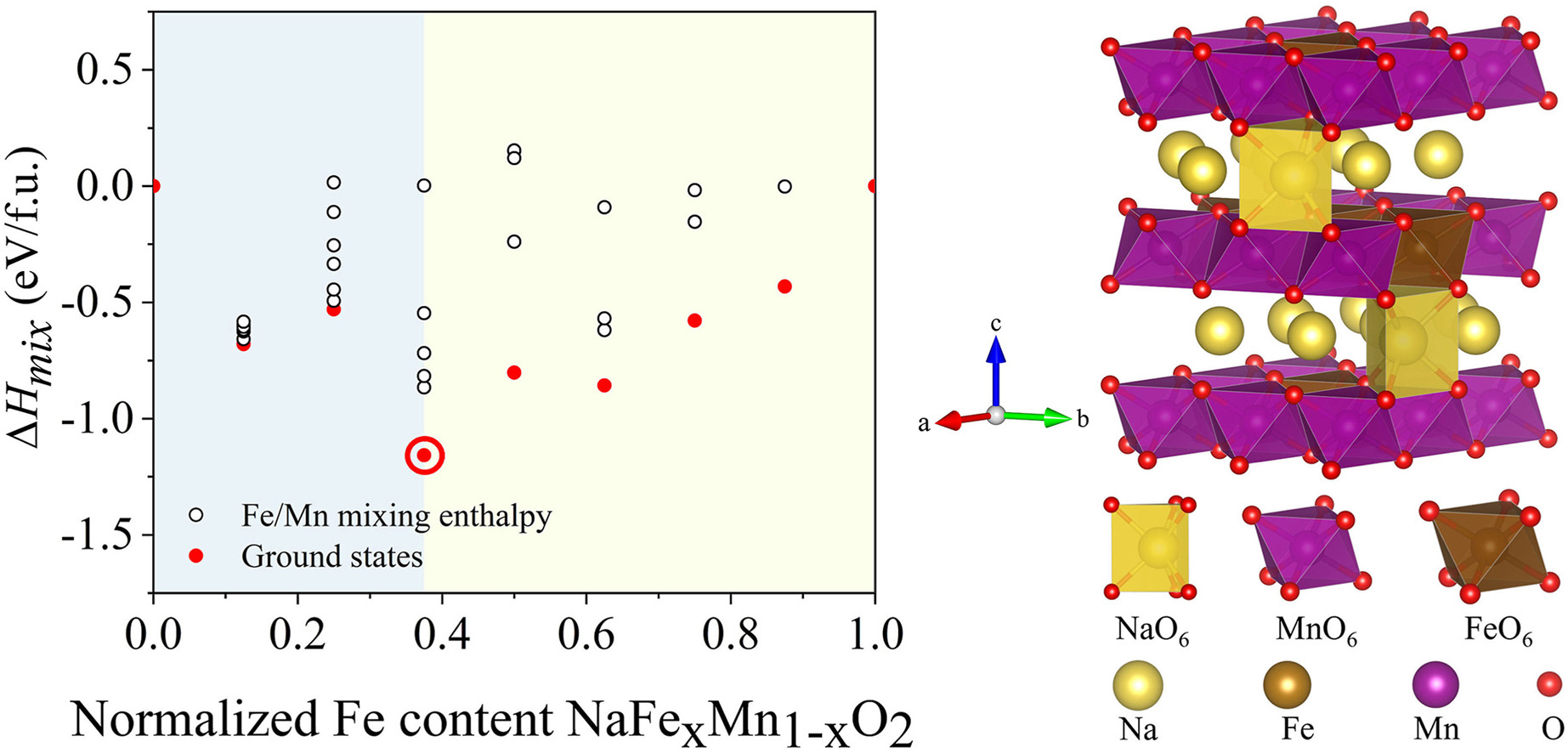
This article conducts first-principles calculations to initially explore the construction of two configurations, NaFeO2 (NFO) and NaMnO2 (NMO), and studies the mixing enthalpies under different Fe–Mn ratios. The results indicate that NaFe3/8Mn5/8O2 (NFMO) exhibits the most thermodynamically stable structure. Subsequent calculations on the mixing enthalpies and volume changes during the sodium extraction process for NFO, NMO, and NFMO configurations are presented, along with the partial density of states (PDOS) and Bader charges of transition metals (TM) and oxygen. These calculations reveal the synergistic mechanism of Fe and Mn. Fe and Mn can engage in more complex electron exchanges during sodium extraction, optimizing the internal electron density distribution and overall charge balance, thereby stabilizing the crystal structure and reducing the migration of Fe3+ to the sodium layers during deep sodium extraction. The interaction between Fe's 3d electrons and Mn's 3d electrons through the shared oxygen atoms'2p orbitals occurs in the Fe–Mn–O network. This interaction can lead to a rebalancing of the electron density around Mn3+ atoms, mitigating the asymmetric electron density distribution caused by the d4 configuration of the lone Mn3+ and suppressing the Jahn-Teller effect of Mn3+. Moreover, the synergistic effects between Fe and Mn can provide a more balanced charge distribution, reducing extreme changes to the charge state of oxygen atoms and decreasing the irreversible oxygen release caused by anionic redox reactions during deep sodium extraction, thereby enhancing the material's stability. This in-depth study of the interaction mechanism at the microscopic level when co-doping Fe and Mn offers valuable insights for the rational design and development of high-performance cathode materials.