• Pd-nanoparticles were decorated on UiO-66-NH2 to reduce diffusion energy barrier.
• Pd-nanoparticles were decorated on UiO-66-NH2 could increase the hydrogen spillover effect.
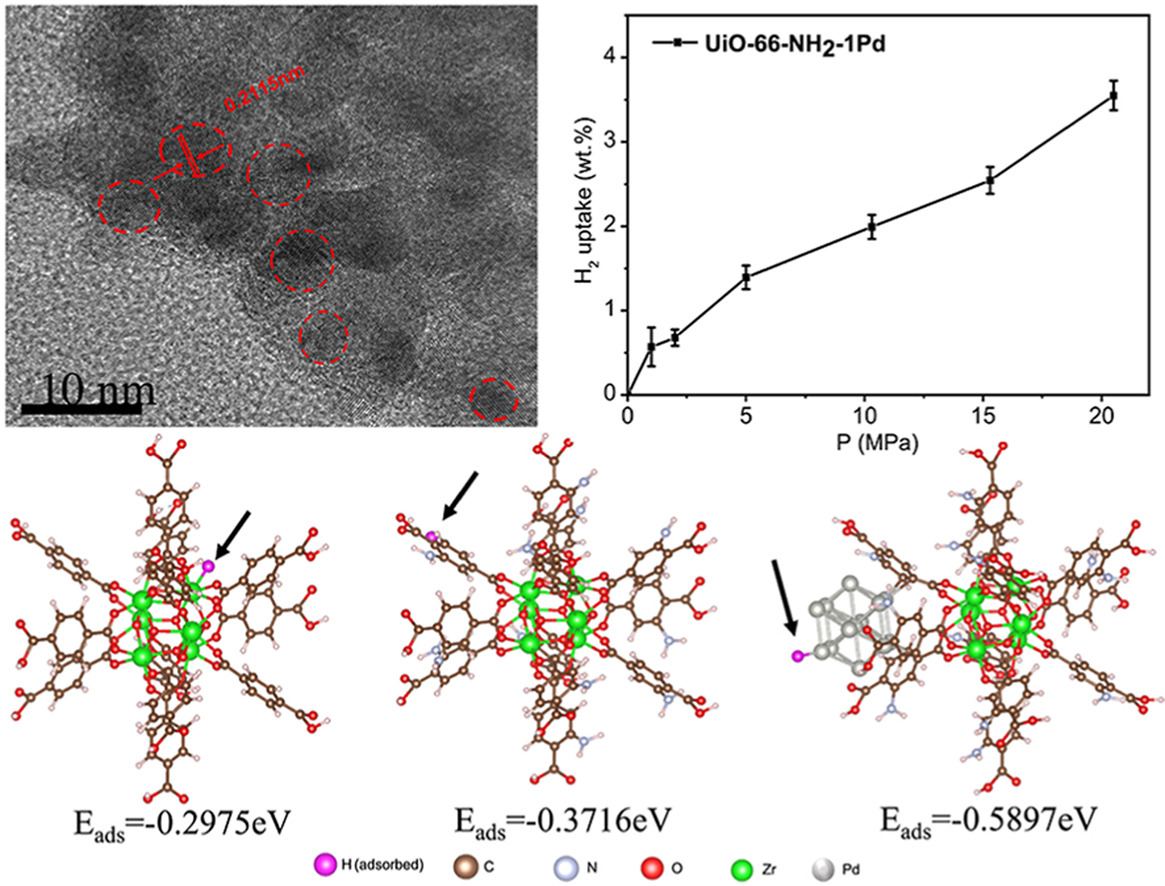
• The hydrogen up taken in UiO-66-NH2-1Pd was 3.7 wt% under 20 MPa at 298 K.
• DFT calculations show that Hads of UiO-66-NH2-Pd was much lower than that of UiO-66-NH2 and UiO-66.
To improve room-temperature hydrogen storage, palladium (Pd) nanoparticles were innovatively decorated by carbon bridge onto the amino-group functioned Zr-terephthalate metal-organic framework (MOF) UiO-66 to reduce the diffusion energy barrier and then improve the hydrogen spillover effect. Powder X-ray diffraction shows broad Pd peak and retained UiO-66-NH2 integrity after Pd decoration. The hydrogen uptake capacity show that UiO-66-NH2-Pd exhibits best hydrogen storage performance than UiO-66-NH2 and pristine UiO-66. The hydrogen up taken in Pd decorated UiO-66 (UiO-66-NH2-1Pd) was close to 4 wt% under 20 MPa at room temperature. Density functional theory (DFT) calculations show that hydrogen adsorption energy of UiO-66-NH2-Pd was −0.5897 eV, which was much lower than that of UiO-66-NH2 (−0.3716 eV) and UiO-66 (−0.2975 eV). Ultimately, Pd decorated NH2 group functioned UiO-66 enable improve storage capacities through hydrogen spillover under ambient conditions which could satisfy the demand for sustainable energy, especially for the long-term storage energy media.