-
Volumes 84-95 (2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 94
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Electrochemical performance of Mo6+-doped LiMn2O4 cathode materials.
• Mo6+-doped LiMn2O4 cathode materials prepared using a simple solid-phase sintering method.
• LMO-0.01Mo exhibits excellent cycling stability and rate performance.
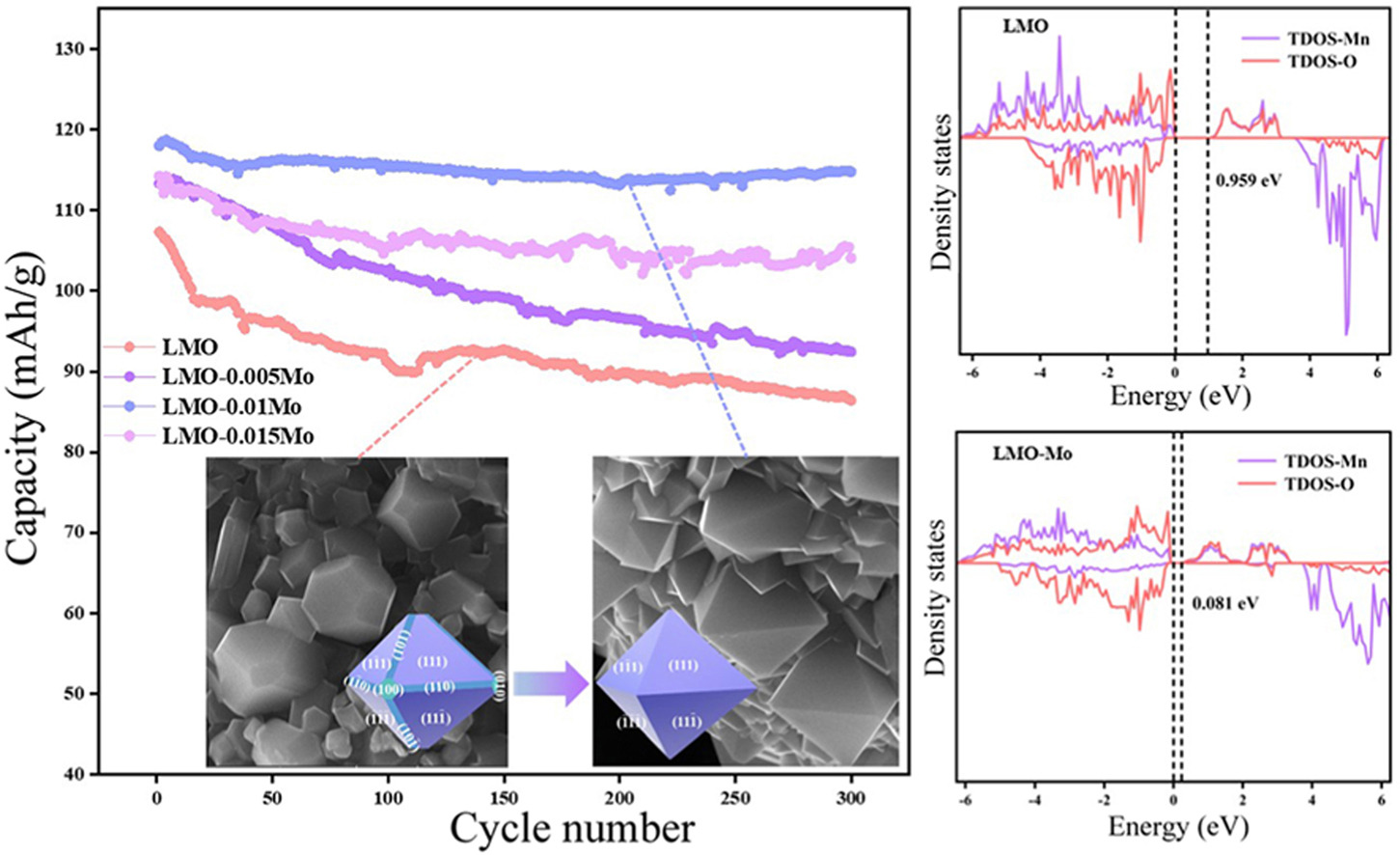
The Jahn-Teller effect and the dissolution of Mn are significant factors contributing to the capacity degradation of spinel LiMn2O4 cathode materials during charging and discharging. In this study, Mo6+-doped polycrystalline octahedral Li1.05Mn2-xMoxO4 (x = 0, 0.005, 0.01, 0.015) cathode materials were prepared by simple solid-phase sintering, and their crystal structures, microscopic morphologies, and elemental compositions were characterized and analyzed. The results showed that the doping of Mo6+ promoted the growth of (111) crystalline facets and increased the ratio of Mn3+/Mn4+. The electrochemical performance of the materials was also tested, revealing that the doping of Mo6+ significantly improved the initial charge/discharge specific capacity and cycling stability. The modified sample (LMO-0.01Mo) retained a reversible capacity of 114.83 mA h/g with a capacity retention of 97.29% after 300 cycles. Additionally, the doping of Mo6+ formed a thinner, smoother SEI film and effectively inhibited the dissolution of Mn. Using density-functional theory (DFT) calculations to analyze the doping mechanism, it was found that doping shortens the Mn-O bond length inside the lattice and increases the Li-O bond length. This implies that the Li+ diffusion channel is widened, thereby increasing the Li+ diffusion rate. Additionally, the modification reduces the energy band gap, resulting in higher electronic conductivity.