-
Volumes 84-95 (2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 94
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
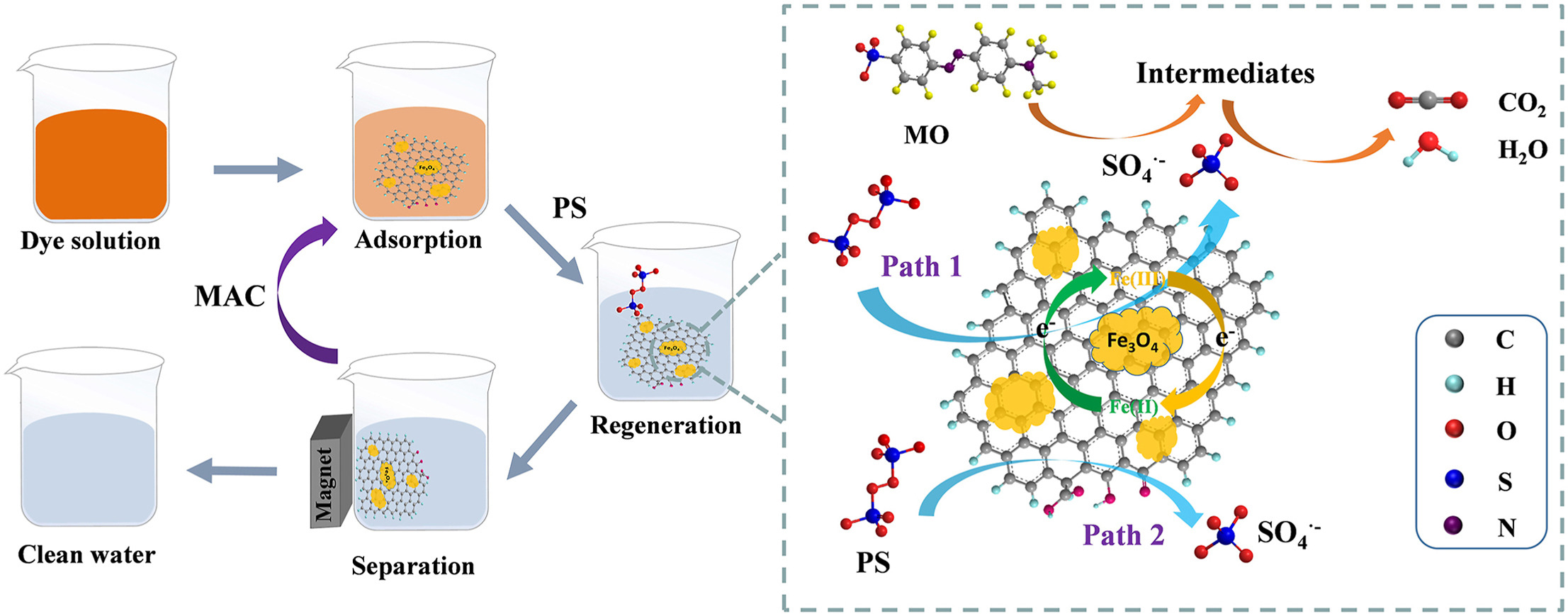
• MAC used for the removal of MO via adsorption and Fenton-like degradation.
• MAC can be effectively separated from water using magnet.
• MO removal exceeded 90% within 60 min after 5 cycles of regeneration.
Water pollution caused by organic dyes is a critical environmental issue. Although activated carbon (AC) is commonly used for dye adsorption, its effectiveness is limited by challenges in separation and regeneration. To address these limitations, a convenient recyclable magnetic activated carbon (MAC) was fabricated via co-precipitation and calcination method, serving as adsorbent and catalyst for methyl orange (MO) removal through a Fenton-like degradation process. Characterization techniques, including XRD, FTIR, SEM and TEM, confirmed that Fe3O4 nanoparticles (10–20 nm) were uniformly dispersed on AC surface. The MAC maintaining a high surface area (997 m2/g) and pore volume (0.795 cm3/g) and exhibited superparamagnetic properties with a saturated magnetization of 5.52 emu/g, enabling effective separation from aqueous solutions by magnet. Batch adsorption studies revealed that MO adsorption onto MAC followed pseudo-second-order kinetic and Freundlich isotherm model, with a maximum adsorption capacity of 205 mg/g at 25 °C. Thermodynamic analysis showed that the adsorption process was spontaneous and endothermic. Simultaneous degradation of MO and in-situ regeneration of MAC were achieved via Fenton-like reaction using sodium persulfate (PS). Under a PS concentration of 9 mmol/L, the MO removal efficiency near 95% after 60 min, with a total organic carbon (TOC) reduction of 83.1%. The reaction of Fe3O4 and oxygen functional groups on AC surface with PS facilitated the generation of 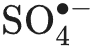
, thereby enhancing catalytic degradation of MO. The degradation efficiency improved as the temperature increased from 25 °C to 45 °C. Cycle tests demonstrated that the MO removal efficiency of MAC remained above 90% after 5 cycles of regeneration. Overall, this study highlights the potential of MAC for efficient removal of organic dyes from water through the coupling of adsorption and Fenton-like degradation, providing a promising solution for addressing water pollution challenges.