• Cells based on highly loaded LiCoO2 and artificial graphite electrodes are evaluated.
• Reasons for different cyclabilities at −10, 0, and 25 °C are revealed.
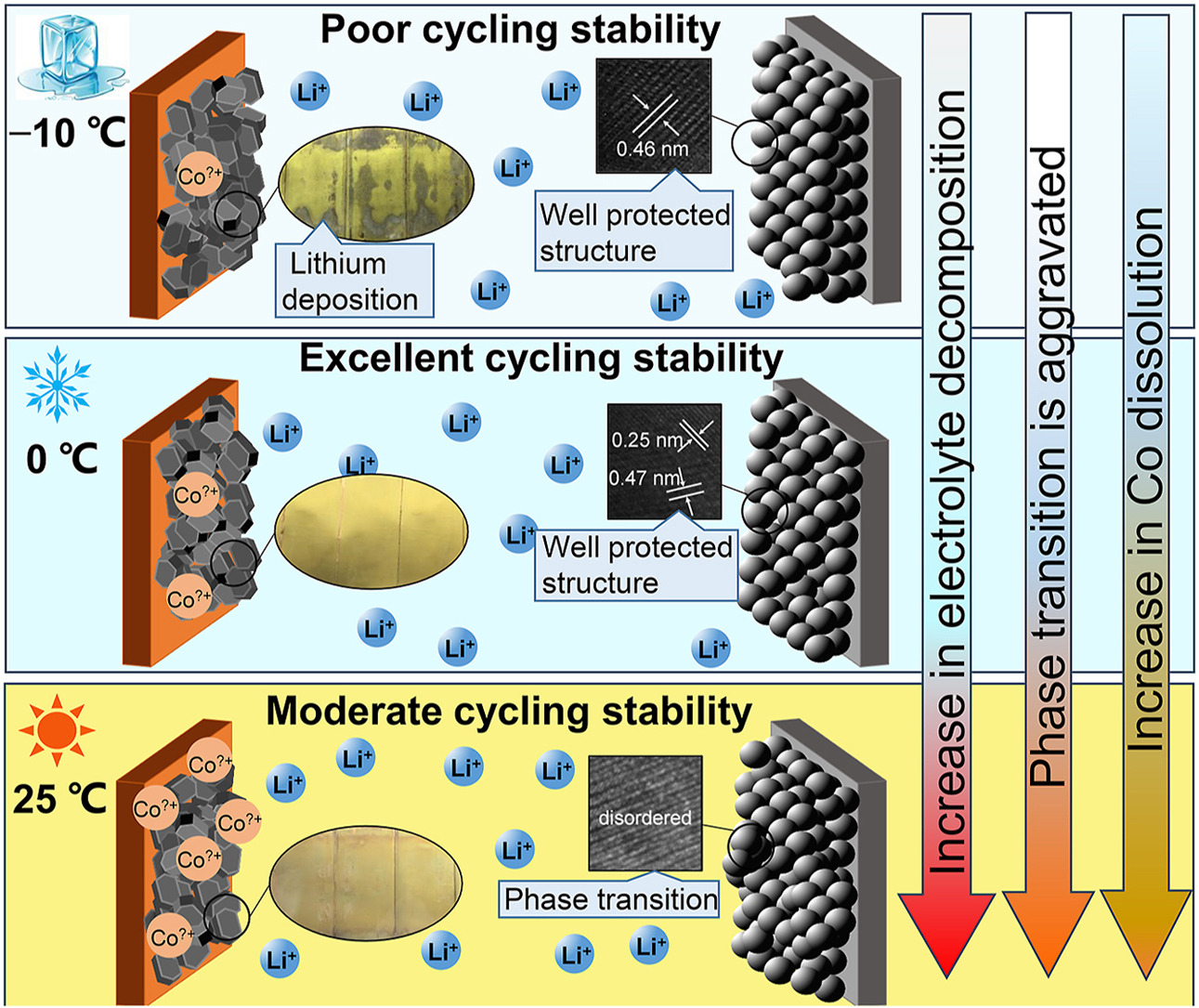
• Severe lithium deposition results in capacity dropping at −10 °C.
• No lithium deposition and slight side reactions contribute to excellent cyclability at 0 °C.
• Electrolyte breakdown and electrode structural disruption jointly cause capacity decay at 25 °C.
Although a few studies reveal the reasons of poor charge-discharge abilities for lithium-ion batteries based on LiNixMnyCo1-x-yO2 at low temperature, there are still some practical issues worthy of further investigation. For instance, how the side reactions affect the cyclabilities of commercial LiCoO2/artificial graphite (LCO/AG) cells at different low temperatures, and are the synergistic effects between the side reactions similar to that at room temperature? To answer the issues, the performances of a ⁓3 Ah LCO/AG pouch cell at different temperatures and C-rates are studied. Results illustrate that the obvious increase in charge transferring impedance especially in AG anode at low temperature causes large polarization, then reducing charge-discharge ability and even yielding lithium deposition at −10 °C and 0.5C under 3−4.45 V. Different from at room temperature, the side reactions such as electrolyte decomposition and electrode structural evolution reduce significantly at low temperature, which contribute to an excellent cyclability after 500 cycles at 0 °C. Instead, a series of chain reactions cause a relative lower cyclability at 25 °C. Lithium deposition is slight after 5 cycles at −10 °C, but become considerably severe after 20 cycles and cause rollover failure of capacity. All these results deepen the understanding on mechanisms for different behaviors of LCO/AG cells at low temperature and provide optimization direction.