• The SiO2 layer effectively restrains Pt nanoparticle sintering during high-temperature calcination.
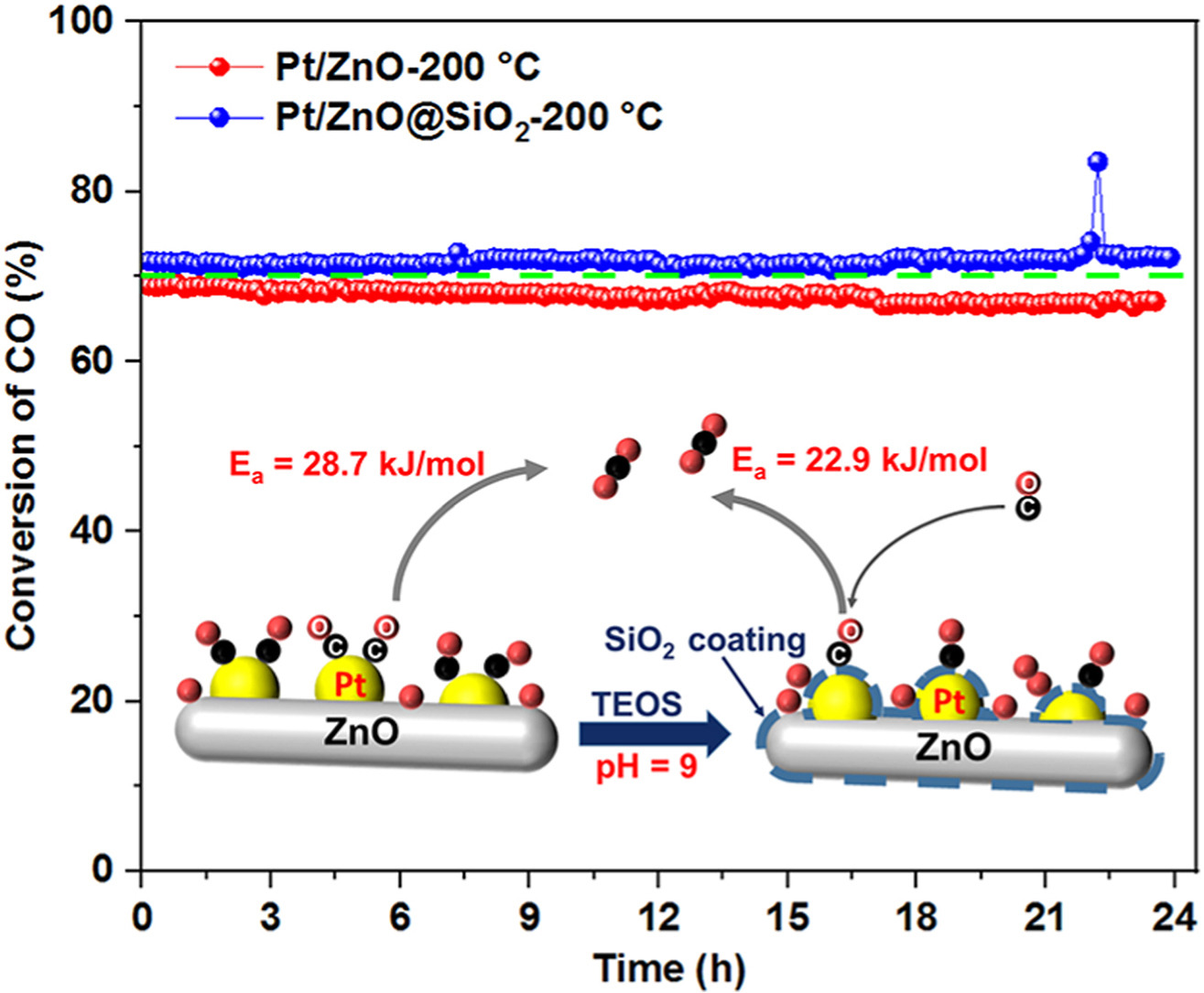
• The SiO2 coated on Pt-based catalyst enhances the oxidation activity and stability of low-temperature CO oxidation.
• The SiO2 layer is cost-effective and readily available.
The catalytic oxidation of carbon monoxide (CO) to carbon dioxide (CO2) is an effective way to eliminate the harmful effects of CO, with catalysts playing a crucial role in this process. Although Pt-based catalysts have been widely used for CO oxidation, the low-temperature activity and thermal stability still need to be improved. In this study, a Pt/ZnO@SiO2 composite structure was constructed by coating Pt/ZnO catalysts with a thin SiO2 layer. The influence of SiO2 overcoating layer on the sintering behavior of Pt nanoparticles (NPs) and on the catalytic performance of the Pt catalyst for CO oxidation was investigated in detail. And the results were compared with those without SiO2 overcoating layer. Investigations found that the SiO2 coating layer effectively inhibited the sintering of Pt NPs at high temperatures, enhancing the thermal stability. In addition, the SiO2 overcoating layer improved the catalytic activity of the Pt-based catalyst by inducing higher concentration of oxygen vacancies on the catalyst surface as well as weakening the CO adsorption, which could enhance the adsorption and activation ability of oxygen. Meanwhile, the presence of SiO2 overcoating layer improved the catalytic stability during CO oxidation reaction. This work provides an important reference for the design and development of supported Pt-based catalysts with excellent thermal stability and catalytic activity for CO oxidation.