•Numerical model of a single particle was developed.
•Decarbonization performance of a single particle was investigated.
•Effects of particle size,gas velocity,and CO2 mass fraction were studied.
•Correction formula for surface CO2 concentration was proposed.
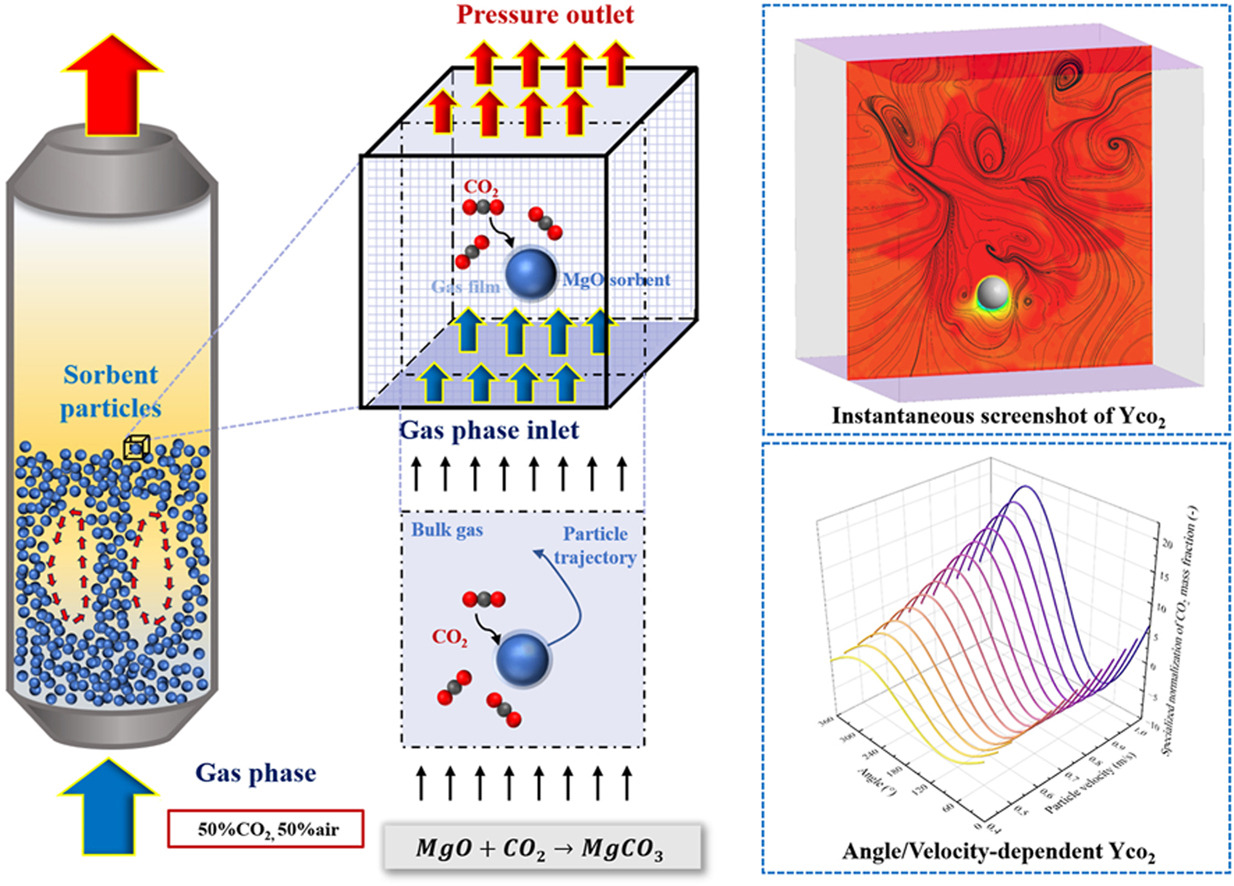
To obtain a deeper understanding of the mass transfer between MgO-based sorbent particles and gaseous reactants (CO2), it is essential to investigate the mass transfer characteristics of a single moving sorbent particle since most previous researches focused on the removal efficiency of the whole flow field and ignored the behavior of individual particles. Currently, most studies assumed that the reactant (CO2) concentration across the particle surface is uniform based on the average concentration within the grid, while the reactant concentration on the particle surface changes as the particle moves. In this study, the gas-solid mass transfer between CO2 and a moving MgO-based particle was investigated. The results indicated that the motion state and velocity of the particles significantly impact the CO2 removal dynamics and noticeable differences in the CO2 concentration gradient could be observed around the particle. Increasing the reaction temperature, enhancing the CO2 mass fraction at the inlet, appropriately increasing the gas velocity, and selecting an appropriate particle size can significantly enhance the reaction rate,thereby improving the CO2 removal efficiency. The correction formula for surface CO2 concentration and mass transfer reaction rate was also proposed based on the particle's velocity and direction of movement. Based on the correction formula,more detailed guidance for optimizing reactor design could be obtained and the findings provide theoretical guide for designing CO2 removal reactors.