• Heat transfer in the dilute region of fluidized viscous powders was studied.
• Dilute phase solid holdup remains stable at various gas velocities under cold state.
• Heat transfer coefficients in the CaO/Ca(OH)2 powders fluidization were obtained.
• Average experimental error is about 7.14 % using Geldart-A heat convection model.
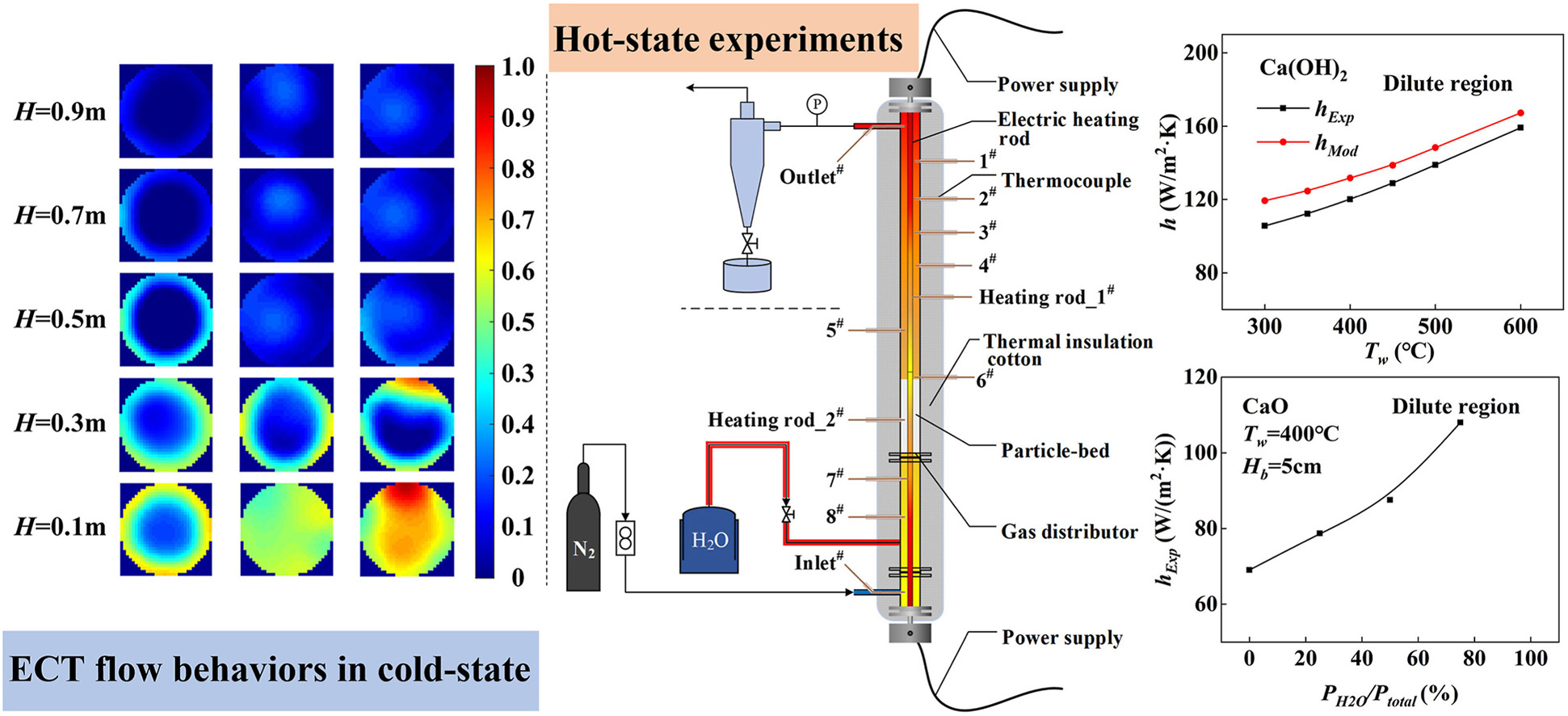
Thermochemical energy storage (TCES) based on the reversible hydration/dehydration of CaO/Ca(OH)2 is emerging as a promising method for harnessing sustainable and renewable energy sources. In this study, a fluidized bed reactor was utilized to investigate the flow and heat transfer characteristics of micron-sized CaO/Ca(OH)2 particles under dilute-phase conditions. Detailed experiments were carried out to measure particle concentration, temperature distribution, and heat transfer coefficients across a range of operating parameters. The results demonstrated that the heat transfer coefficient from the heated wall to the fluidized particles increases significantly with both reaction temperature and vapor partial pressure. Furthermore, the cluster renewal model was employed to predict the heat transfer behavior, achieving a low average relative error compared to the experimental data. These findings enhance the fundamental understanding of fluidized-bed-based CaO/Ca(OH)2 TCES processes and offer practical guidelines for optimizing large-scale, high-temperature energy storage systems in support of intermittent renewable energy applications.