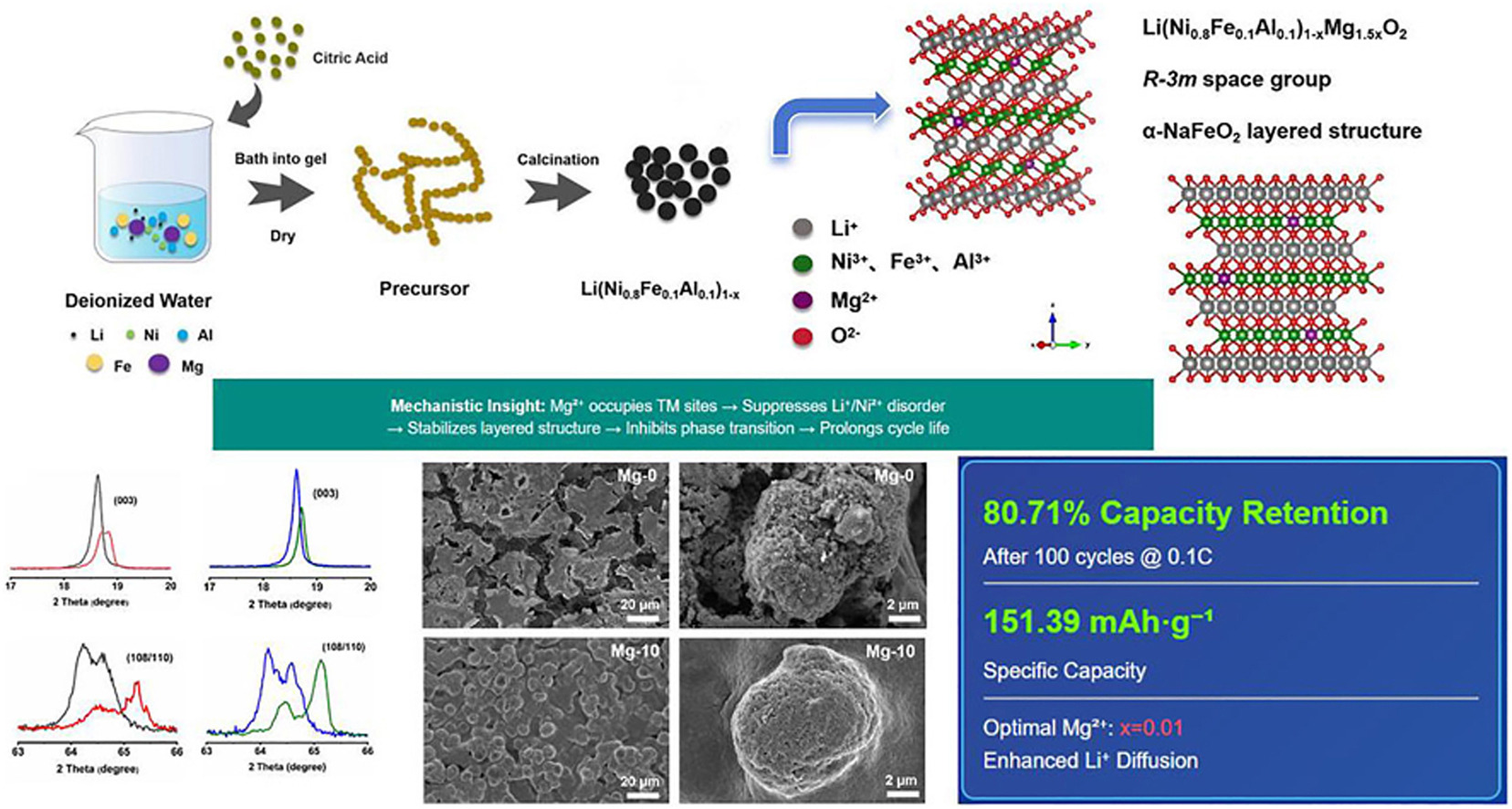
• Mg2+ is preferred to replace the transition metal (TM) site to enhance the stability of the structure.
• Optimal Mg-10 cathode exhibits superior rate capability and long-term cyclability.
• Mechanistic insights reveal Mg2+ stabilizes layered frameworks and widens Li + diffusion channels.
• This work offers valuable insights into the sustainable development of nickel-rich cobalt-free cathode materials.
In recent years, cobalt has emerged as a critical limiting factor in the production chain of the lithium-ion battery industry. The increasing demand for electric vehicles has made the dependence on cobalt in lithium-ion batteries a significant challenge for environmental sustainability. To address the problem, a new class of cobalt-free materials has been introduced, called lithium iron aluminium nickel oxides (NFA) cathode materials, with the general formula Li(Ni0.8Fe0.1Al0.1)1-xMg1.5xO2 (x = 0, 0.005, 0.01, 0.015). In this work, a series of cobalt-free materials were synthesized via sol-gel processes, and variations in magnesium content were explored to investigate their compositional landscape. Electrochemical performance evaluations revealed that Mg2+ doping significantly improved the electrochemical properties of the material. Among them, samples prepared with 1.5 mol% Mg2+ doping (i.e. the value of 0.01 for x) exhibited the best cycling capacity. After 100 cycles at 0.1C, the capacity retention rate was found to be 80.71 %, with a specific capacity of 151.39 mAh g−1, demonstrating remarkable rate capability and cycling stability. Although still in the nascent stages of the investigation, these cathodes hold potential as candidates for the next generation of cobalt-free lithium-ion batteries.