- Volumes 96-107 (2025)
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
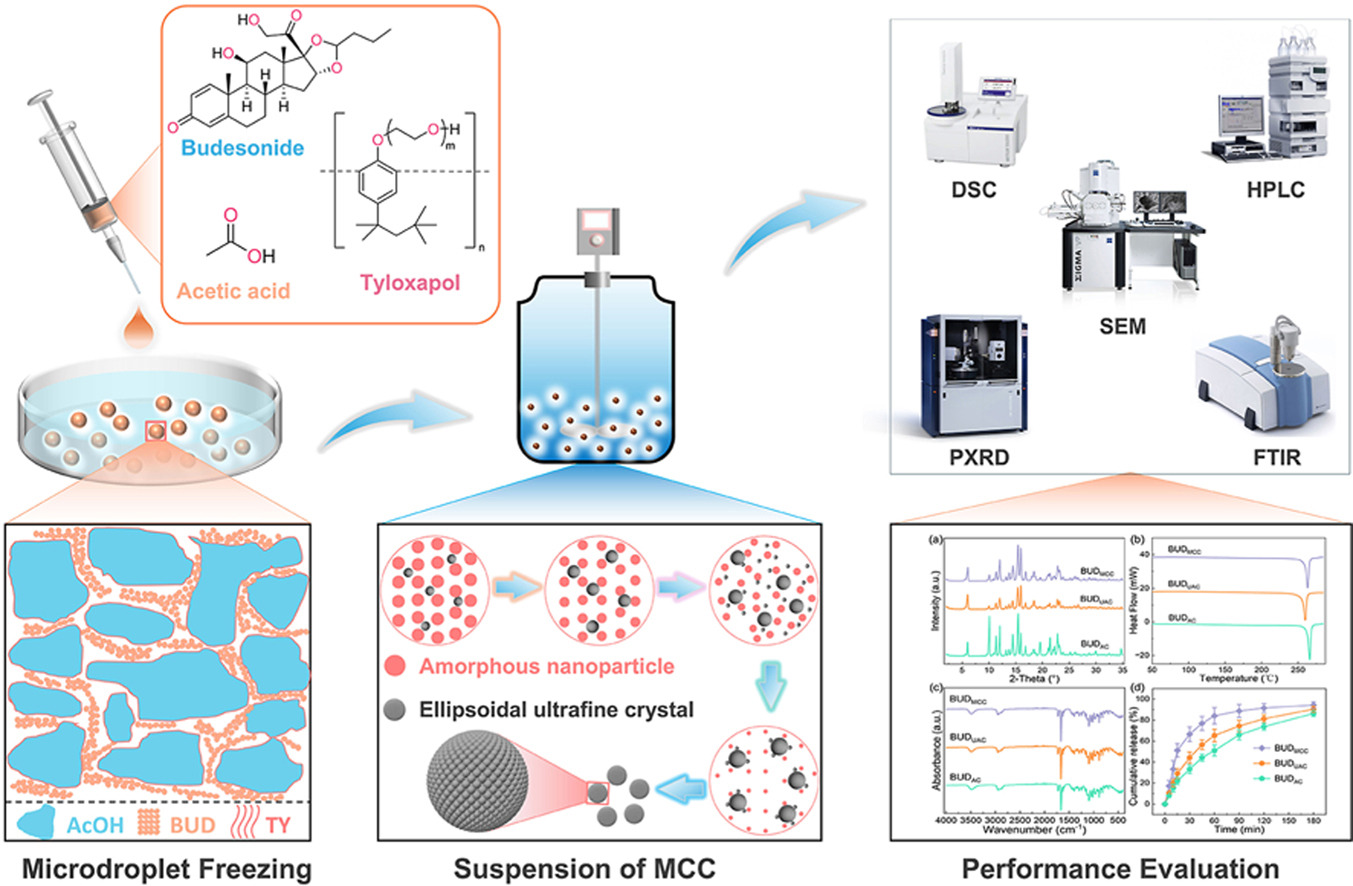
• Microdroplet cryo-crystallization was proposed to prepare ultrafine crystals.
• Engineering budesonide ultrafine crystals with superior size, shape, and crystallinity.
• Revealing the phase transformation mechanism of nanoprecursors during suspension process.
• MCC-BUD microcrystals exhibit superior dissolution performance compared to those produced by conventional methods.
Inhalation therapies are pivotal for treating pulmonary diseases, yet their efficacy critically depends on the physicochemical properties of drug particles. This study introduces a novel microdroplet cryo-crystallization (MCC) technique to fabricate inhalable budesonide (BUD) particles. The MCC process combines rapid cryogenic freezing of drug-loaded microdroplets in liquid nitrogen, followed by additive-guided suspension crystallization in an anti-solvent environment. Cryogenic freezing suppresses molecular mobility and prevents aggregation, preserving uniform solute distribution. Subsequent controlled crystallization in the anti-solvent system enables precise tailoring of nanoparticle morphologies while avoiding supersaturation-driven amorphization. Systematic optimization identified MCC conditions yielding BUD ultrafine crystals with a volume median diameter of 3.0 μm, >94 % sphericity, >98 % crystallinity, and minimal hygroscopicity (<0.5 %). Compared to conventional air-jet milled BUD (∼90 % crystallinity and ∼3 % hygroscopicity), the MCC-engineered particles exhibit significantly improved physicochemical stability and dissolution performance (94 % in 180 min). The MCC strategy decouples cryogenic freezing and phase transformation, avoiding top-down limitations (e.g., milling-induced amorphization) and bottom-up issues (uncontrolled nucleation/aggregation) to achieve scalable and highly precise production of inhalable drug particles.