• Aeromonas caviae shows natural potential in biodegradation due to diverse enzymatic pathways.
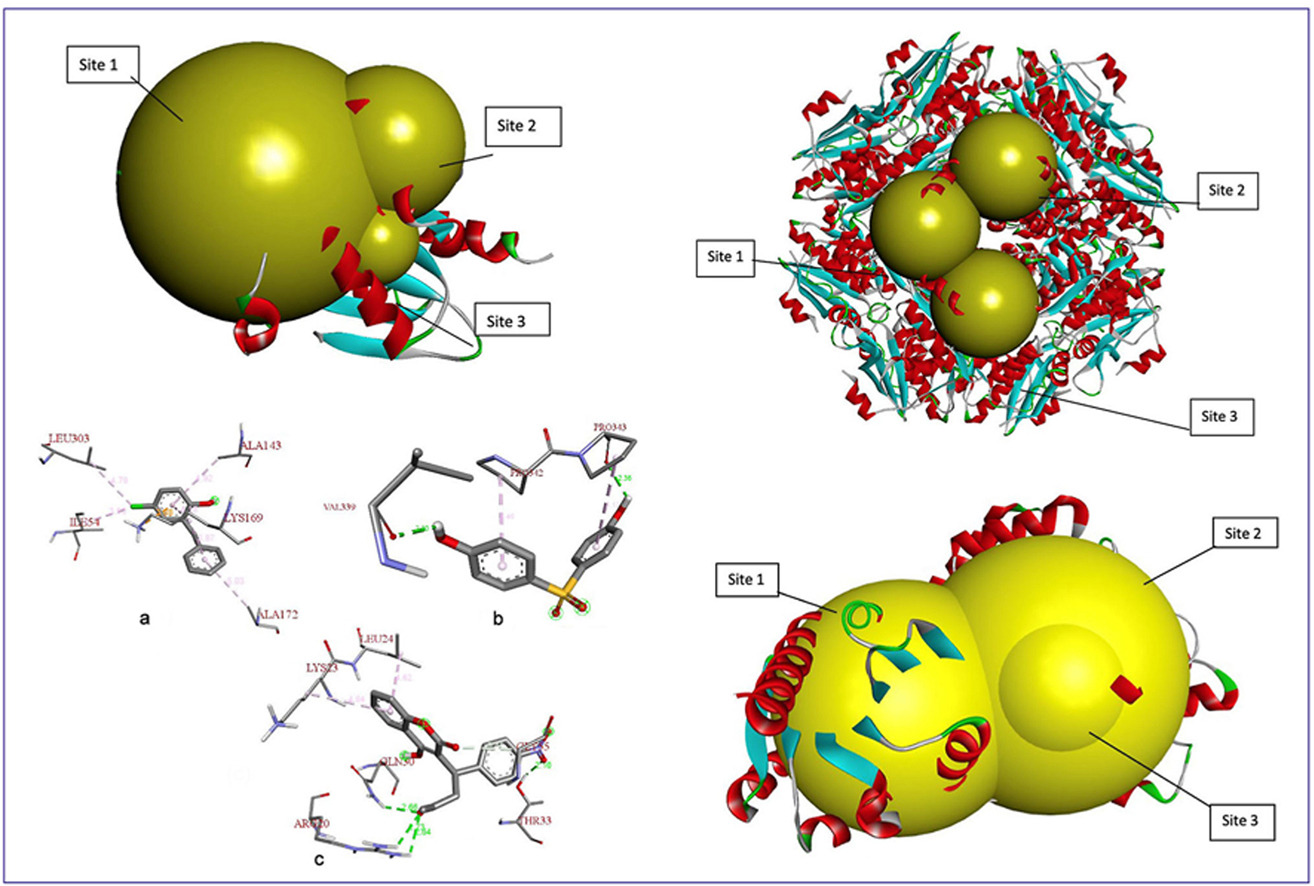
• Docking shows synergy of beta-ketoadipate enol-lactone hydrolase and muconate cycloisomerase in phenol breakdown.
• Ligand-protein docking revealed effective interactions in the hybrid biodegradation enzyme model.
• Bioengineered hybrid enzyme showed better docking scores and structural similarity to known homologs.
• Enzyme bioengineering enhanced the biodegradation potential of marine-derived phenolic compounds.
Toxic aromatic compounds are widespread environmental pollutants posing significant ecological and health risks due to their persistence and toxicity. Aeromonas caviae is a promising microbial candidate for biodegradation owing to its diverse enzymatic arsenal. This study investigates the cooperative roles of two key enzymes from A. caviae, beta-ketoadipate enol-lactone hydrolase, which is involved in ring-opening hydrolysis, and muconate cycloisomerase, which catalyzes the isomerization of muconate intermediates, in degrading 15 selected toxic compounds using computational methods. Enzyme stability analysis via ExPASy ProtParam indicated an instability index below 40, confirming structural stability. Homology models were constructed and validated with high-quality scores. Molecular docking revealed Acenocoumarol as the compound with the highest binding affinity (−7.8 kcal/mol). Protein-ligand interaction analysis identified key residues involved in Pi-Pi and hydrogen bonding interactions critical for catalysis. Furthermore, a bioengineered hybrid enzyme model demonstrated improved binding precision and structural similarity to homologous proteins. These findings highlight the potential application of A. caviae enzymes in the bioremediation of toxic pollutants. Future experimental validation and enzyme engineering could further enhance their catalytic efficiency for sustainable environmental detoxification.