-
Volumes 96-107 (2025)
-
Volume 105
-
Volume 104
-
Volume 103
Pages 1-314 (August 2025)
-
Volume 102
Pages 1-276 (July 2025)
-
Volume 101
Pages 1-166 (June 2025)
-
Volume 100
Pages 1-256 (May 2025)
-
Volume 99
Pages 1-242 (April 2025)
-
Volume 98
Pages 1-288 (March 2025)
-
Volume 97
Pages 1-256 (February 2025)
-
Volume 96
Pages 1-340 (January 2025)
-
Volume 105
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
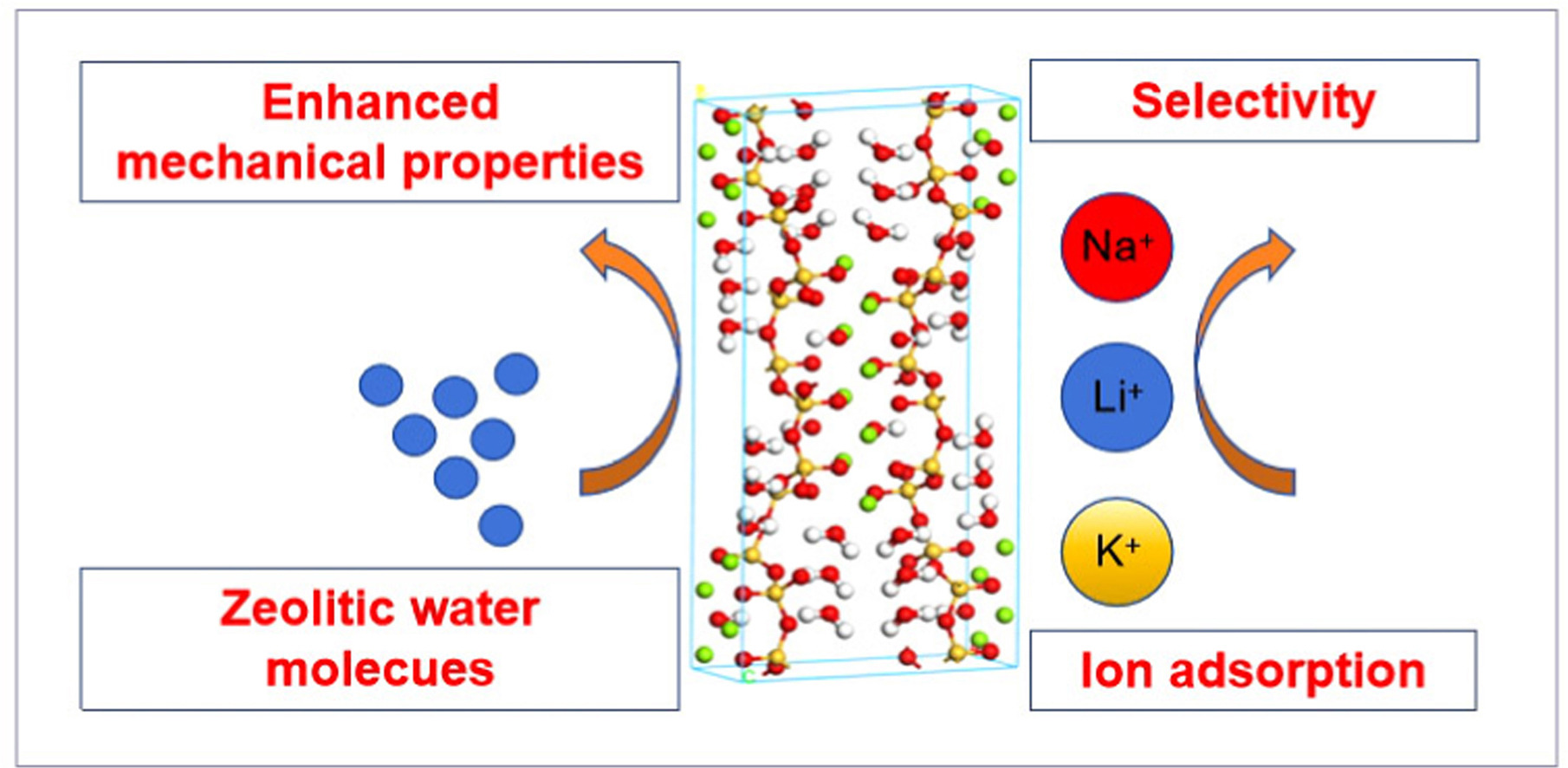
• Mechanical properties of sepiolite are calculated by molecular dynamics simulation.
• Number of zeolitic water molecules greatly affects mechanical properties of sepiolite.
• Li+, Na+ and K+ ions show different adsorption behaviors on surface of sepiolite.
• Ionic selectivity of sepiolite is in order of Na+ > Li+ > K+.
Sepiolite, a hydrated magnesium silicate known for its fibrous structure, is extensively utilized in water treatment for its ability to adsorb a variety of metal ions. Despite its widespread utilization, the mechanical properties of sepiolite, which are crucial for its practical applications, have been largely overlooked. To address this gap, this paper employs molecular dynamics (MD) simulation to explore the mechanical characteristics of sepiolite, including structural parameters, bulk modulus, and Young's modulus. The research findings reveal that zeolitic water molecules contribute positively to enhancing the mechanical properties of sepiolite. As the number of zeolitic water molecules rises, the Young's modulus values in the x and y directions show a clear increasing trend. However, the quantity of zeolitic water molecules does not significantly affect the Young's modulus in the z direction or the Poisson's ratio. Additionally, the paper assesses the adsorption capacity of sepiolite for Li+, Na+, and K+. The results indicate that as ion concentration rises, the absolute number of ions adsorbed onto the surface increases, yet the corresponding adsorption percentage shows a notable decline. Based on the adsorption capacity, the ion selectivity order of sepiolite is determined to be: Na+ > Li+ > K+. The MD simulations elucidate the adsorption mechanism by which alkali metal ions interact with and adhere to the sepiolite surface.