-
Volumes 96-107 (2025)
-
Volume 105
-
Volume 104
-
Volume 103
Pages 1-314 (August 2025)
-
Volume 102
Pages 1-276 (July 2025)
-
Volume 101
Pages 1-166 (June 2025)
-
Volume 100
Pages 1-256 (May 2025)
-
Volume 99
Pages 1-242 (April 2025)
-
Volume 98
Pages 1-288 (March 2025)
-
Volume 97
Pages 1-256 (February 2025)
-
Volume 96
Pages 1-340 (January 2025)
-
Volume 105
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
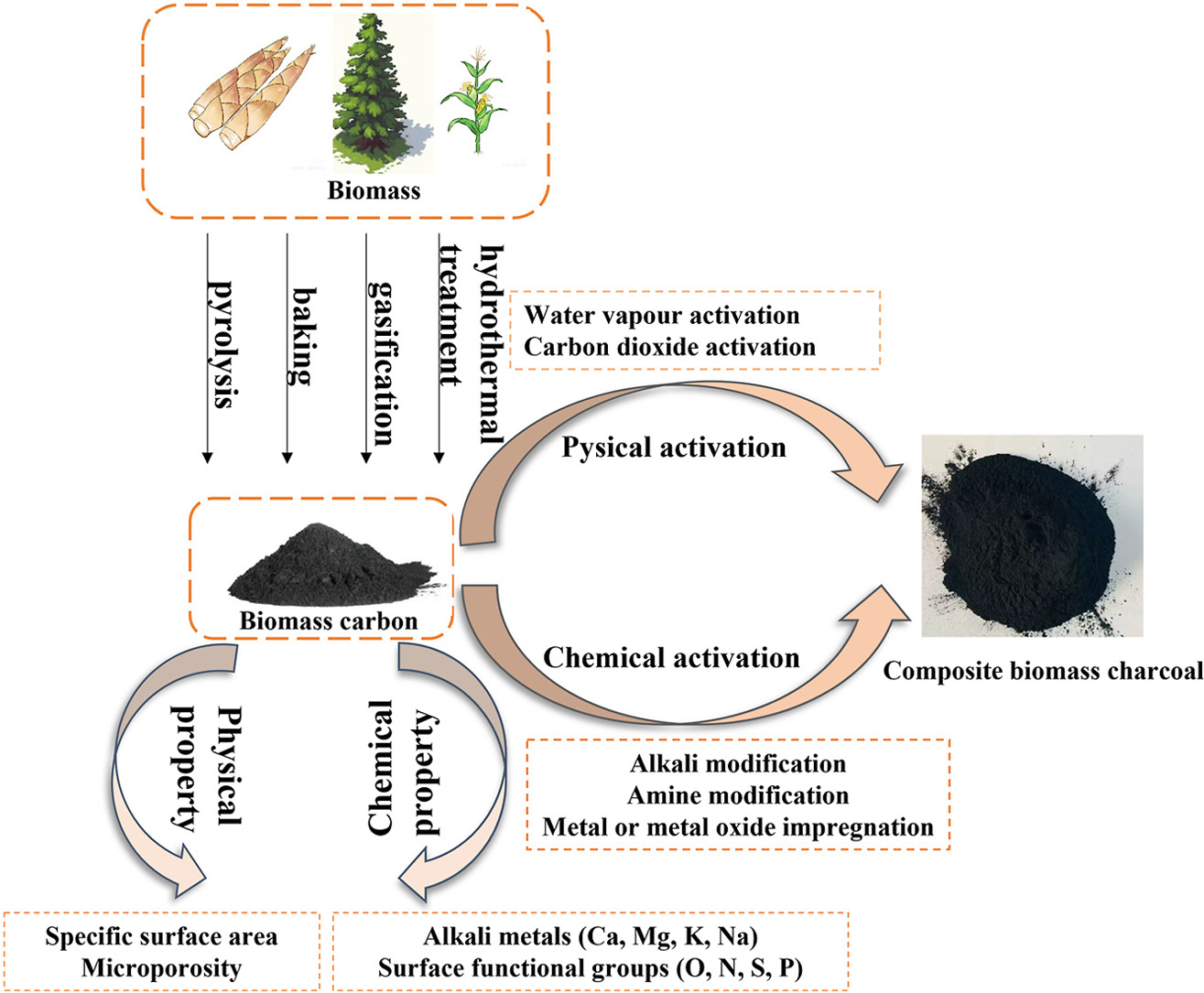
• Sources, physicochemical properties, preparation, and modification of biomass-based CO2 adsorbents are reviewed.
• Surface alkali metals and N/S-containing functional groups enhance biomass char alkalinity and CO2 affinity.
• Physical activation (CO2, steam) and chemical modification (KOH, amine grafting, metal oxide impregnation) boost adsorption capacity.
• Future research should focus on in-situ mechanisms, heteroatom co-doping, and scalable production.
Excessive CO2 emissions pose a severe environmental threat, driving interest in carbon capture technologies. Biomass-derived activated carbon (BAC) emerges as a promising adsorbent due to its renewable feedstocks, cost-effectiveness, and tunable properties. This review comprehensively analyzes recent advances in BAC for CO2 capture. Key findings indicate that agricultural/forestry wastes are optimal feedstocks, while alkali/alkaline earth metals and N/S-containing functional groups enhance surface alkalinity and CO2 affinity. Pyrolysis is identified as the preferred preparation method for optimizing pore structure. Physical activation (CO2, steam) and chemical modification (KOH/NaOH, amine grafting, metal oxide impregnation) significantly improve porosity and adsorption capacity. Notably, N-doping increases CO2 uptake by 31.6–55.2 %, and microporous volume (0.59–0.71 cm3/g) is critical for performance. However, challenges include high energy consumption during KOH activation, feedstock variability impacting consistency (>22 % ash content differences), competitive adsorption from flue gas impurities (>30 % capacity loss), and metal leaching risks. Future research should prioritize in-situ mechanistic studies, heteroatom co-doping, and scalable production techniques to advance industrial deployment.